Researchers continue to document an increasing number of acute and chronic drug effects of Hyperbaric Oxygen Therapy (HBOT). Acutely, HBOT corrects hypoxia [oxygen deprived], reduces edema, augments WBC-mediated bacterial killing, inhibits an aerobic bacteria, and profoundly decreases reperfusion injury. In chronic wounding HBOT induced effects are trophic: fibroblast proliferation, collagen deposition, epithelialization, and angiogenesis. The latter process is the basis for HBOT generated wound healing and the topic of this HBO on the Avenue.

ANGIOGENESIS, or new blood vessel growth, is critical to wound healing. In normal wound management with minimal tissue destruction angiogenesis occurs without problems at the wound edge where a steep oxygen gradient exists. The stimulus for angiogenesis is hypoxia at the wound edge that causes various growth factors to be released from wound macrophages. This same hypoxia is responsible for retinopathy in newborns and preemies after abrupt withdrawal of supplemental oxygen and in newborn animals subjected to hypoxic environments. Hypoxia is similarly present in chronic or non-healing wounds, but the difference is that the oxygen gradient is very shallow. While no one has defined the exact slope of the shallow gradient, i.e. the distance over which oxygen reduction occurs in a non-healing wound, it is the usual underlying pathophysiology in most non-healing chronic wounds. Besides large vessel revascularization, to date only one therapy has been shown to consistently correct the shallow oxygen gradient and induce angiogenesis: HYPERBARIC OXYGEN THERAPY.
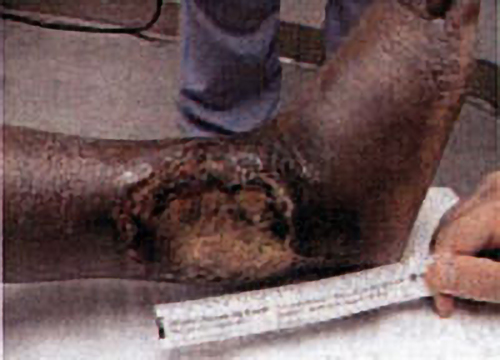
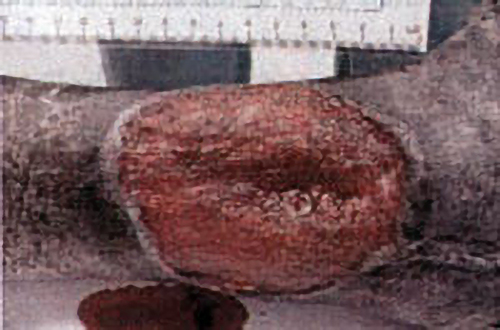
The best model so far developed to study shallow perfusion gradient wounds and the one in which HBOT’s angiogenesis effects have been unequivocally demonstrated is irradiated tissue. External beam radiation causes a well-defined stereotypic delayed thrombosis of small blood vessels that is maximal at the center of the beam and tapers at the edges. Marx (1) exploited this wound in animals and humans to show that HBOT caused a progressive angiogenesis at the wound margin by generating a steep oxygen gradient with intermittent repetitive HBOT. Over a course of about 30 treatments new vessel growth infiltrated the wound and achieved pO₂’s of about 85% of control tissue. Similar HBOT angiogenesis has been achieved in animals by Manson (2), Rohr (3), Meltzer (4), Nemiroff (5), Zhao (6), and others. This is the underlying basis of all HBOT in chronic wounding and accounts for the ability to heal diabetic foot wounds, arterial insufficiency ulcers, traumatic ischemic wounds, bums, and other devascularized wounds, providing major arterial supply is not severely decreased. On reverse side is an example of HBOT’s angiogenesis capability.
- Marx RE, et al. Amer J Surg, 11/90; 160:519-524.
- Manson PN, et al. Surg Forum, 1980; 31:564-6.
- RohrS, et al. Joint Meeting on Diving and Hyperbaric Medicine, 3rd Swiss Symposium, XVIIIth Annual Meeting of EUBS, 9/15- 19/1992, Basel, Switzerland, 112-115.
- Meltzer T, Myers B. The Amer Surg, 12/ 86; 52(12):659-662.
- Nemiroff PM, Lungu AP. Surg Forum, 1987; 38:565-567.
- Zhao LL, et al. Arch Surg, 1994; 129:1043-49
Angiogenesis: The Key to HBOT Healing of a Wound
CASE PRESENTATION
Paul Harch, MD
The patient is a 44 y. o. insulin dependent diabetic female with a 10 month non-healing wound of the right lower leg. The patient sustained a fracture of the right distal tibia / fibula in 10/99 which was treated with ORIF[Open Reduction Internal Fixation (ORIF) is a method of surgically repairing the fractured bone]. The fibular skin wound failed to heal and in 2/00 she underwent split thickness skin grafting with near complete take. A small open wound persisted until 7/00 when she traumatized the wound, resulting in a cellulitis and enlargement that required surgical debridement. The wound continued to deteriorate, prompting referral for hyperbaric oxygen therapy evaluation.