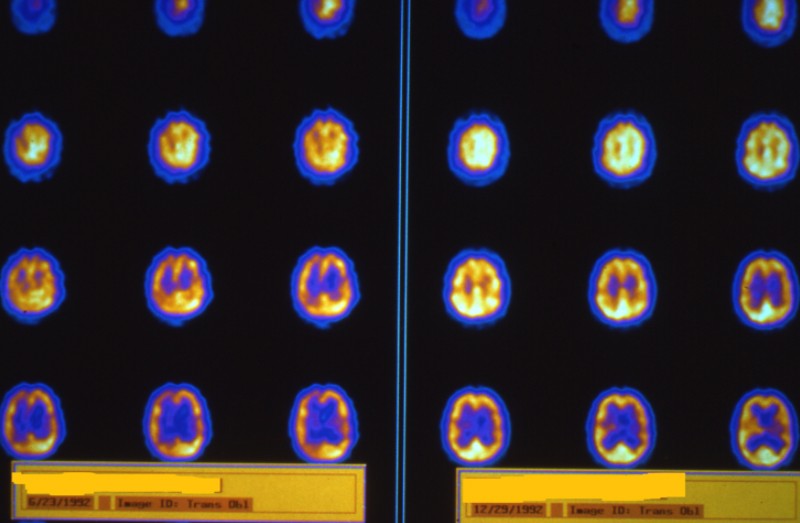
Hyperbaric Application to COVID-19 Pulmonary Infection
March 10, 2020Hyperbaric Oxygen for COVID-19 Report
March 24, 2020Hyperbaric oxygen therapy for mild traumatic brain injury persistent postconcussion syndrome: a randomized controlled trial
PubMed Published March 13, 2020
Paul G. Harch 1 *, Susan R. Andrews 2, Cara J. Rowe 3, Johannes R. Lischka 4, Mark H. Townsend 5, Qingzhao Yu 6, Donald E. Mercante 7
- Department of Medicine, Section of Emergency and Hyperbaric Medicine, Louisiana State University Health Sciences Center, New Orleans, LA, USA
- Department of Medicine and Psychiatry, School of Medicine, Louisiana State University Health Sciences Center, Metairie, LA, USA
- CaTS Clinical Translational Unit, Tulane University School of Medicine, LA, New Orleans, LA, USA
- Family Physicians Center, Marrero, LA, USA
- Louisiana State University-Ochsner Psychiatry Residency Training Program, Department of Psychiatry, Louisiana State University Health Sciences Center, New Orleans, LA, USA
- School of Public Health, Louisiana State University Health Sciences Center, New Orleans, LA, USA
*Correspondence to: Paul G. Harch, MD, [email protected], [email protected]: 0000-0001-7329-0078 (Paul G. Harch)
Abstract
Persistent postconcussion syndrome (PPCS) after mild traumatic brain injury (mTBI) is a significant public health and military problem for which there is limited treatment evidence. The aim of this study was to determine whether forty 150 kPa hyperbaric oxygen therapies (HBOTs) can improve symptoms and cognitive function in subjects with the PPCS of mTBI, using a randomized controlled crossover design with 2-month follow-up. Sixty-three civilian and military subjects with mTBI/PPCS were randomized to either 40 HBOTs at 150 kPa/60 minutes, once daily, 5 days per week in 8 weeks or an equivalent no-treatment control period. The Control Group was then crossed over to HBOT. Subjects underwent symptom, neuropsychological, and psychological testing, before and after treatment or control with retesting 2 months after the 40th HBOT. Fifty subjects completed the protocol with primary outcome testing. HBOT subjects experienced significant improve- ments in Neurobehavioral Symptom Inventory, Memory Index, Automated Neuropsychological Assessment Metrics, Hamilton Depression Scale, Hamilton Anxiety Scale, Post-Traumatic Stress Disorder Checklist, Pittsburgh Sleep Quality Index, and Quality Of Life after Brain Injury compared to the Control Group. After crossing over to HBOT the Control Group experienced near-identical significant improve- ments. Further improvements were experienced by both groups during the 2-month follow-up period. These data indicate that 40 HBOTs at 150 kPa/60 minutes demonstrated statistically significant improvements in postconcussion and Post-Traumatic Stress Disorder symptoms, memory, cognitive functions, depression, anxiety, sleep, and quality of life in civilian and military subjects with mTBI/PPCS compared to controls. Improvements persisted at least 2 months after the 40th HBOT. The study was registered on ClinicalTrials.gov (NCT02089594) on March 18, 2014 and with the U.S. Food and Drug Administration under Investigational New Drug #113823. The Institutional Review Boards of the United States Army Medical Research and Materiel Command Office of Research Protections Human Research Protection Office and the Louisiana State University School of Medicine (approval No. 7381) approved the study on May 13, 2014 and December 20, 2013, respectively.
Key words: chronic brain injury; hyperbaric oxygen therapy; neurobehavioral symptom inventory; neuropsychological testing; neuroreha- bilitation; persistent postconcussion syndrome; post-traumatic stress disorder; randomized controlled trial; symptoms; traumatic brain injury
doi: 10.4103/2045-9912.279978
How to cite this article: Harch PG, Andrews SR, Rowe CJ, Lischka JR, Townsend MH, Yu Q, Mercante DE. Hyperbaric oxygen therapy for mild traumatic brain injury persistent postconcussion syndrome: a randomized controlled trial. Med Gas Res. 2020;10(1):8-20.
Funding: This study was supported by U.S. Army Medical Research and Materiel Command Fort Detrick, No. W81XWH-10-1-0962, and Joe W. and Dorothy Dorsett Brown Foundation.
Introduction
Mild traumatic brain injury (mTBI)/persistent postconcussion syndrome (PPCS) is a significant public health and military problem. In 2013 there were 2.8 million emergency department visits, hospitalizations, or deaths in the United States due to TBI,1 75% of which are estimated to be mild TBI.2 When non-hospital non-emergency department visits for head trauma are included there were an additional 1.16 million adult (18–64 years old)3 and 845,000 pediatric cases,4 comprising approximately 50% of all head trauma cases in the U.S. In total there appears to be at least 4.8 million TBI cases annually in the U.S., 4.1 million of which are mild TBI. This figure is further increased by military service members and the elderly non-emergency department/hospital TBI subsets and is orders of magnitude higher worldwide.
Historically, only 15% of mild TBI patients are diagnosed with the PPCS,5 but more recent literature suggests a rate as high as 55%5 for mTBI with loss of consciousness. The longer the symptoms persist the higher the likelihood that they will become permanent. When symptoms persist longer than 3 years the syndrome appears to be permanent.6,7 In a military veteran population nearly 70% of patients entering the Veterans Administration system with a diagnosis of TBI were still receiving treatment 4 years later.7 Treatment has consisted of psychoeducational interventions, cognitive rehabilitation, psychotherapeutic approaches, integrated behavioral health
interventions, and psychoactive medication administration. There is some evidence to support the use of cognitive rehabilitation approaches,8 limited evidence for the other three non-pharmacologic interventions,8 and very little evidence for psychoactive medications.9 This is a pharmacologic study which employed a well characterized biological wound- healing therapy, hyperbaric oxygen therapy (HBOT), to treat the chronic brain wounds of mTBI.10
HBOT is the use of increased atmospheric pressure and hyperoxia as drugs to treat disease pathophysiology11 through gene expression and suppression.12 Treatment effects are a function of dose and timing of intervention in the disease process.13 HBOT doses of 200–300 kPa have been applied to a limited 15 reimbursed acute central nervous system and acute or chronic extremity wound and infection diagnoses in the U.S.14,15 while a much larger list of diagnoses have been treated internationally.16-18 Lesser doses have been used mainly for chronic neurological conditions.13
HBOT has been applied to chronic TBI in animals and humans since 198919-41 with apparent conflicting results.25,27 Various researchers have attributed the different results in mTBI PPCS to mischaracterized sham groups/the effects of different doses of HBOT,11,12,24,42-47 design differences,48 (small sample size, dissimilar outcome measures/populations/sites/ protocol adherence, non-equivalence of group, selection bias),29 ritual experience,28 and placebo/Hawthorne effects.49 Regardless, all of the studies performed at 150 kPa of oxygen in mTBI/PPCS have generated positive data.22,24,26,28,29,39,40 The purpose of this study was to use a randomly assigned Treatment Group versus Control Group design to demonstrate efficacy and confirm or refute the previous experience using the 150 kPa oxygen dose of HBOT.
Subjects and Methods
Full details of the Methods and Protocol are in Additional file 1.
Design
Subjects were randomly assigned to Treatment Group or Control Group; the Control Group then crossed over to receive HBOT following the control period (Figure 1). There was no sham control group in this study. Due to the bioactivity of oxygen and hydrostatic pressure,11,1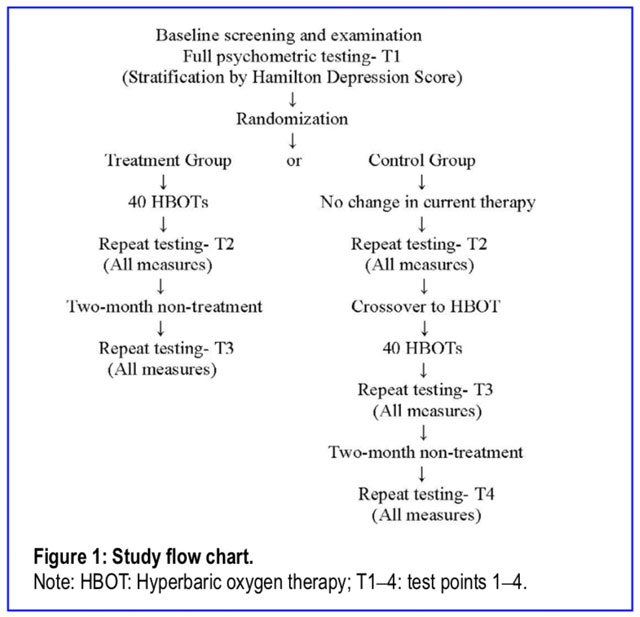 2,50 the two active components of an HBOT,11,12 the requirement of the absence of these two components for a true sham51 HBOT,11,12 and the absence of successful demonstration of a true sham HBOT in the history of clinical HBOT, a first-ever true sham HBOT control group was not attempted in this efficacy trial.
2,50 the two active components of an HBOT,11,12 the requirement of the absence of these two components for a true sham51 HBOT,11,12 and the absence of successful demonstration of a true sham HBOT in the history of clinical HBOT, a first-ever true sham HBOT control group was not attempted in this efficacy trial.
The outcome data was primarily generated by the study neuropsychologist who was blinded to group designation (single-blind). The study was registered on Clinical Trials.gov (NCT02089594) on March 18, 2014 and with the U.S. Food and Drug Administration under Investigational New Drug #113823. The Institutional Review Boards of the United States Army Medical Research and Materiel Command Office of Re- search Protections Human Research Protection Office and the Louisiana State University School of Medicine (approval No. 7381) approved the study on May 13, 2014 and December 20, 2013, respectively. The writing and editing of the article were performed in accordance with the CONsolidated Standards Of Reporting Trials (CONSORT) Statement.
subjects
Subjects were 18–65 year old adults who had experienced one or more blunt or blast mTBIs, as defined by the American Congress of Rehabilitation Medicine mTBI definition,52 that was at least 6 months old (3 months longer than the minimum time limit for definition of PPCS),53 occurred on or after September 11, 2001, resulted in the symptoms of the PPCS54 that developed within 4 weeks after the mTBI, and were continu- ously present through to enrollment. Subjects had to score at least 2255 on the Neurobehavioral Symptom Inventory (NSI)56 and complain of headache, a marker of symptomatic mTBI in both military 57 and civilian populations 58 with equal incidence in blast and blunt mTBI.59
screening procedure and neuropsychological outcome testing
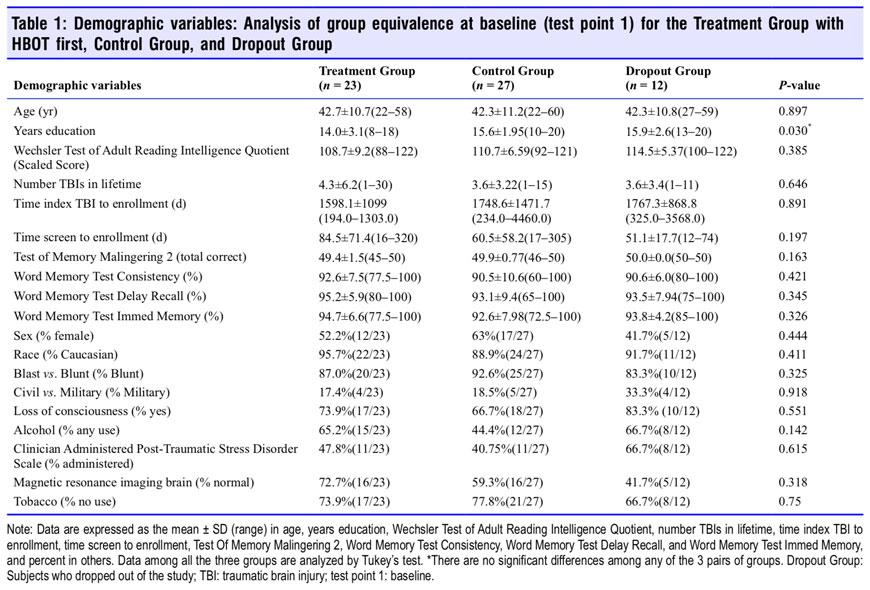
Subjects were screened with the NSI, Michigan Alcohol Screening Test,60 Drug Abuse Screening Test,61 Post-Traumatic Stress Disorder Check List-Military or Civilian (PCL-M or C 4: score less than 50),62 Ohio State TBI Identification Method63 structured interview, Clinician Administered PTSD Scale64 if the PCL was ≥ 50, semi-structured psychiatric evaluation, in- depth medical history by the principal investigator, and effort testing with complete neuropsychological outcome test battery [Test of Memory Malingering,65 Green Word Memory Test,66 Wechsler Test of Adult Reading,67 Hamilton Depression Scale (HAM-D),68 Hamilton Anxiety Scale (HAM-A),69 Wechsler Adult Intelligence Scale (WAIS-IV)70 or Wechsler Abbrevi- ated Scale of Intelligence,71 Wechsler Memory Scale,72 Rey Auditory Verbal Learning Test Delayed Recall (RAVLT),73 Benton Visual Retention Test (BVRT),74 Stroop Test,75 Con- trolled Oral Word Association Test,76 Category Fluency Test (Animals Test),77 Automated Neuropsychological Assessment Metrics (ANAM-4.1 A-1746T Core version),78 Pittsburgh Sleep Quality Index (PSQI),79 and Quality of Life after Brain Injury (QOLIBRI)].80 Subjects were then stratified by the HAM-D score and randomized to either the control (Control Group) or HBOT (Treatment Group) treatment using a block randomization scheme with random block sizes of four, six, or eight implemented in the R programming language.
Postconcussion symptoms were measured using the NSI. Cognitive functions were measured by five categorical vari- ables constructed to reduce the data plus three additional mea- sures (RAVLT-Delayed Recall, the ANAM-4.1, and Benton Visual Retention Test). The five categorical variables were: 1) Working Memory Index, 2) Memory Index, 3) Executive Function Index using T-scores,81 4) Information Processing Speed Index, and 5) General Intellectual Ability (See Addi- tional file 2 for index construction). The behavioral/emotional changes were measured using the HAM-D, HAM-A, PSQI, the QOLIBRI, and the PCL-C or PCL-M. The NSI and Working Memory Index were chosen as co-primary outcomes for the study82-85 and sample size determined by prior data in veterans24 and control group effects.86
Hyperbaric Treatment
Forty treatments at 150 kPa for 60 minutes without air breaks were delivered consecutively in Class B Sechrist Industries (Anaheim, CA, USA) monoplace chambers (Model 2500 or 3200) once a day, 5 days per week.
Statistical Analysis
The primary analysis compared the mean difference in the 14 outcome variables between the two treatment groups (Control and HBOT) from test point 1 to test point 2 using a general linear model and a two-sample t-test. Paired samples t-tests were used to assess changes within treatment groups from test point 1 to each subsequent time point for all 14 outcome variables. For categorical baseline variables chi-squared tests of homogeneity were used to test for differences in proportions across categories among groups. Analyses were performed using SAS 9.4 (SAS, Cary, NC, USA).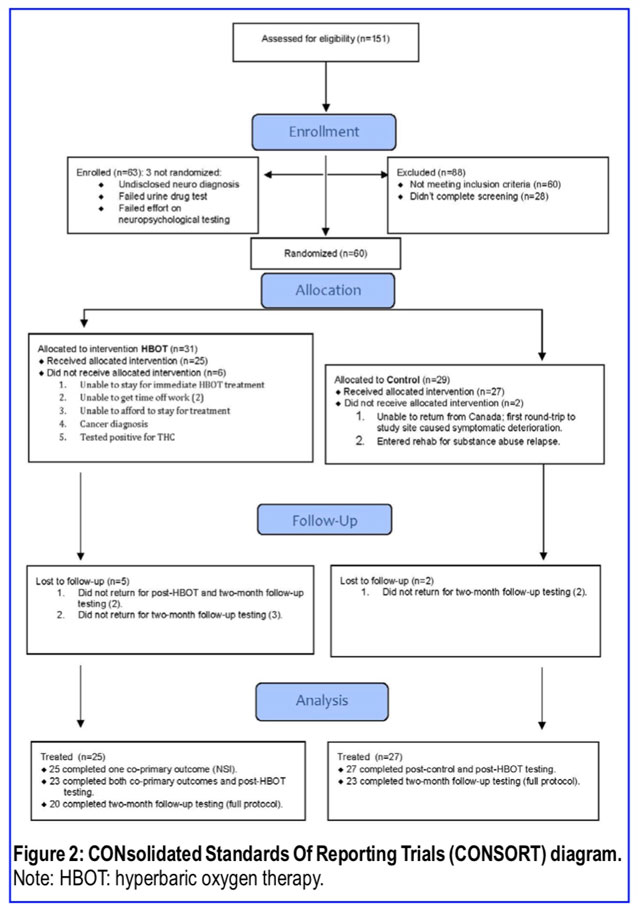
Results
Quantitative analysis of mild traumatic brain injury persistent postconcussion syndrome patients
Recruitment began on May 13, 2014, ended on September 29, 2017, and the last subject completed 2-month follow-up testing on March 5, 2018. Subject enrollment and testing numbers are in Figure 2. Only 12/13 in the Dropout Group were included in the demographic analysis (Tables 1 and 2) since one subject dropped out due to an employer problem, later re-enrolled, and was re-randomized to Control Group. That subject was counted in the Control Group for demo- graphic analysis. Three of the thirteen Dropouts occurred pre-randomization due to an undisclosed post-enrollment discovered disqualifying neurological diagnosis, failed effort testing, and failed urine drug test. Eight of the ten remaining dropouts were in the Treatment Group and two in the Control Group. Four of the eight patients in Treatment Group Dropouts occurred before any treatment was delivered (one could not stay for immediate treatment, two could not obtain work releases for treatment, and one was diagnosed with cancer the day of randomization), one occurred after the third HBOT (financial problems) and one after the first HBOT (principal investigator missed the positive drug test). The other two Treatment Group Dropouts did not report for post-treatment testing. The remaining two Dropouts (Control Group) self-removed from the study due to substance abuse relapse/entry to an inpatient rehabilitation program and deterioration in symptoms upon returning to Canada post- randomization. Five subjects did not complete 40 HBOTs: four due to late fatigue (30, 34, 39, and 39 HBOTs) and one due to a pre-scheduled flight home (39 HBOTs). Thirty Clinician Administered PTSD Scales, based on a PCL over 50 during prescreening, were administered out of the 63 subjects who were enrolled in the study. None were found to have clinical PTSD at the time of enrollment.
Demographics of the sample and dropout analysis
Analyses of group equivalence at baseline for demographic variables and outcome variables are presented for the Treat- ment, Control and Dropout Groups in Tables 1 and 2. Tukey’s Test87 analysis of the two significantly different variables (years of education and NSI) showed no significant difference between any two groups for years of education while the NSI was significantly different between the Control and Dropout Groups. The Dropout subjects had significantly lower symp- tom scores than the Control Group, but the two main study groups (Treatment and Control Groups) did not differ in PPCS complaints on the NSI.
Changes in the outcome after HBOT vs. control period
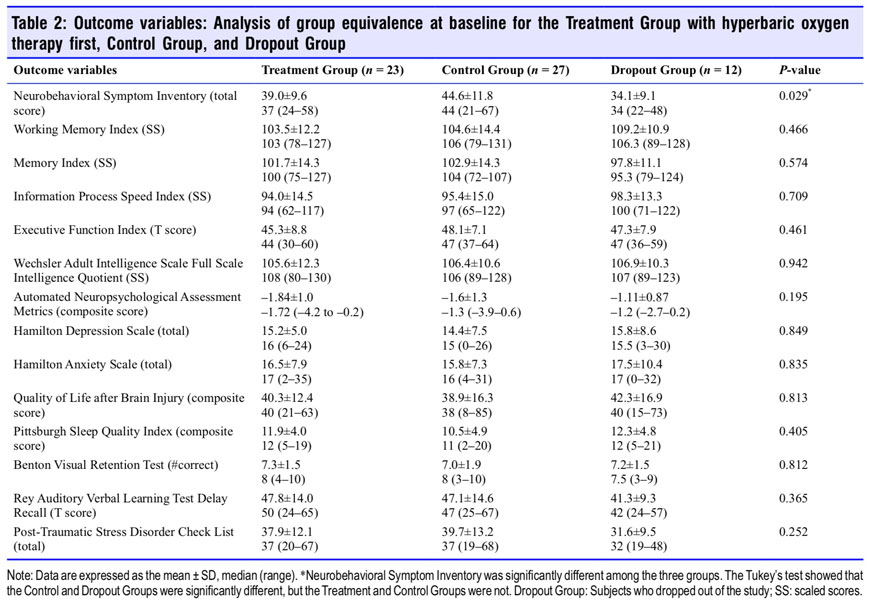
Figure 3 graphs the change in the two co-primary outcome variables (NSI and Working Memory Index) for the control (Control Group) vs. HBOT (Treatment Group) and the proportionate domain changes for NSI in the Treatment Group. The Treatment Group experienced a 26.3-point decrease in the NSI PPCS symptom score compared to a 2.5-point decrease in the Control Group (P < 0.0001). The cognitive domain of the Treatment Group NSI registered the greatest relative improvement with a 19% relative decrease. The difference between the groups in working memory change was not significant. In total eight of the 14 outcome variables were significantly improved in the Treatment Group compared to control (Con- trol Group): PPCS symptoms (NSI), Memory Index, overall cognitive efficiency (ANAM 4), depression (HAM-D), anxiety (HAM-A), quality of life (QOLIBRI), sleep quality (PSQI), and post-traumatic anxiety symptoms (PCL) (Table 3).
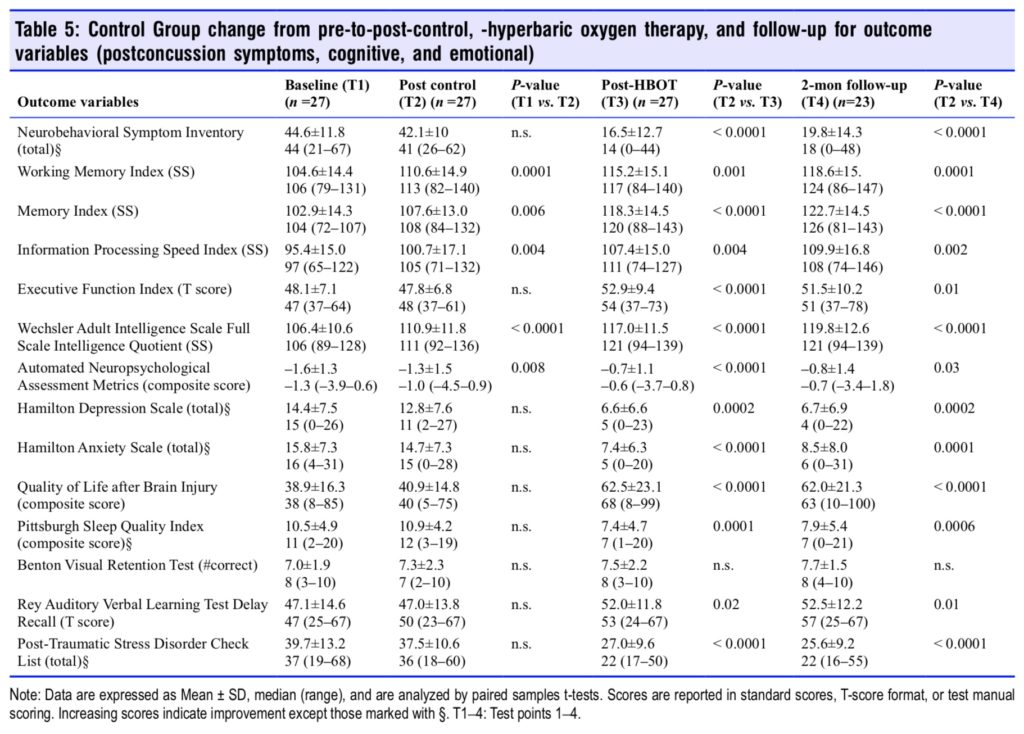
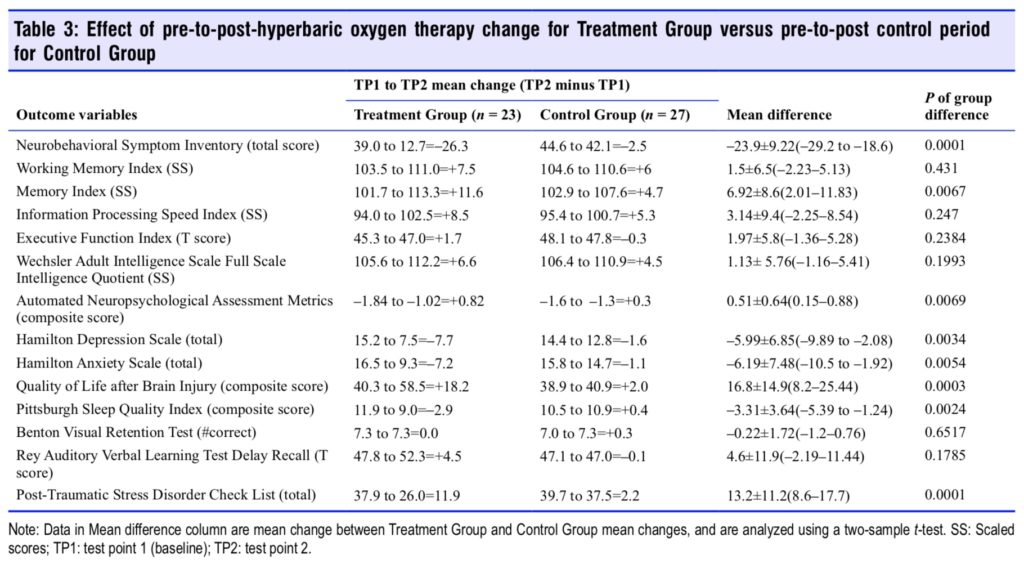
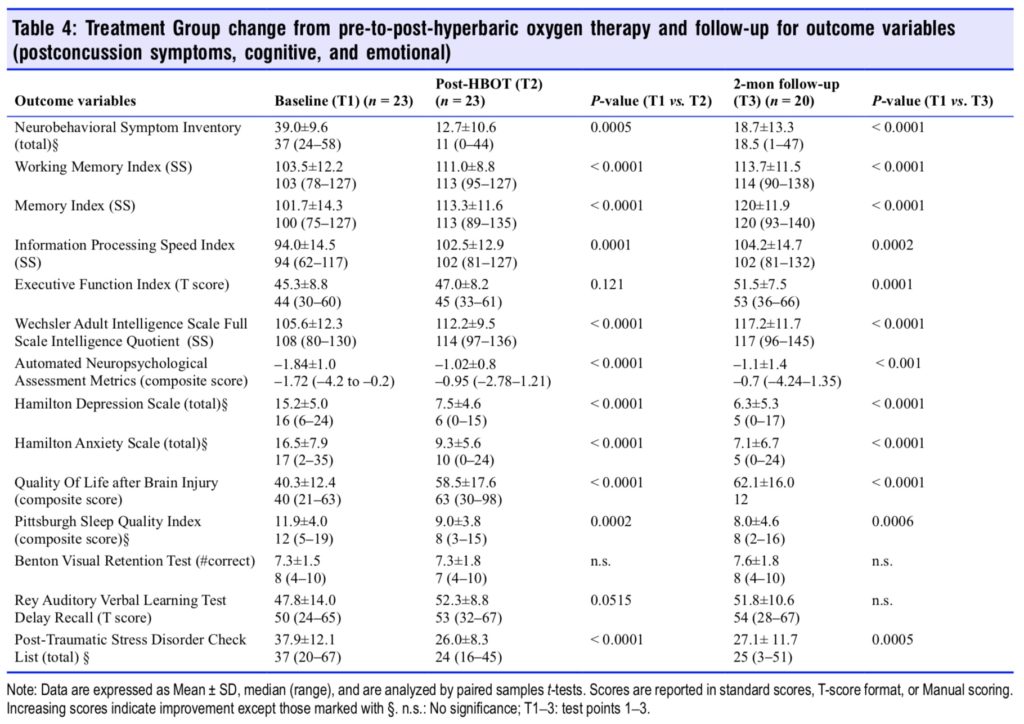
Sequential changes for each group’s 14 outcome variables at all test points are shown in Tables 4 and 5. The Treatment Group experienced significant improvements in 11 of 14 outcome tests after HBOT (Table 4) vs. 5 of 14 tests for the Control Group during the control period; the RAVLT showed a near significant improvement (P = 0.0515) while Executive Function was insignificantly changed in the Treatment Group. After HBOT the Control Group had a significant improvement in 13 out of 14 variables (Table 5) that were nearly identi- cal in magnitude to the same Treatment Group test domain changes. Both groups showed minor changes in the RAVLT while neither group demonstrated improvement in the Benton Visual Retention Test. After HBOT there were no significant differences in any outcome change between groups.
Two months after the last HBOT the two groups maintained or experienced further improvement on most of the outcome variables. Working memory, memory index, information processing speed, executive function, full scale IQ, HAM-D and -A, QOL, and PSQI showed continued improvement for the Treatment Group. The Control Group also maintained their gains but did not have as much improvement. Executive Function and sleep quality were the only two variables that showed a significantly greater improvement for the Treatment Group compared to the Control Group. In sum, both groups showed significant and equal improvement on nearly all out- come variables after treatment by the conclusion of the study.
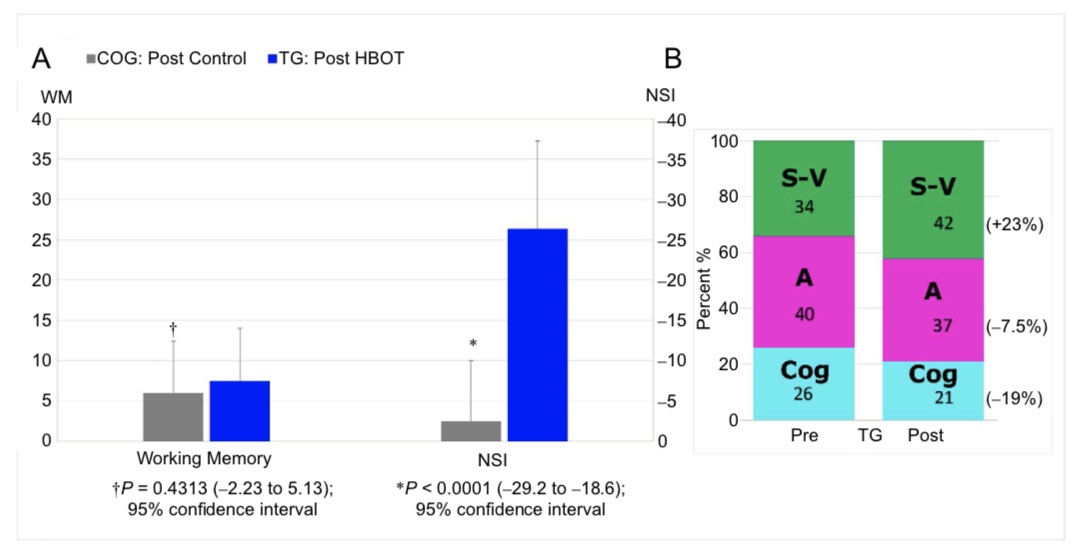
Figure 3: Change in the Neurobehavioral Symptom Inventory (NSI) and Working Memory Index for the Control Group vs. Treatment Group and the proportionate domain changes for NSI in the Treatment Group.
Note: (A) Change in primary outcome measures (post-HBOT minus pre-HBOT or post-control minus pre-control). N = 23 for Treatment Group and 27 for Control Group. (B) Treatment Group domain contributions to total NSI score pre- and post-HBOT. The components of the NSI are the somatic-vestibular (S-V), affective (A) and cognitive (Cog).
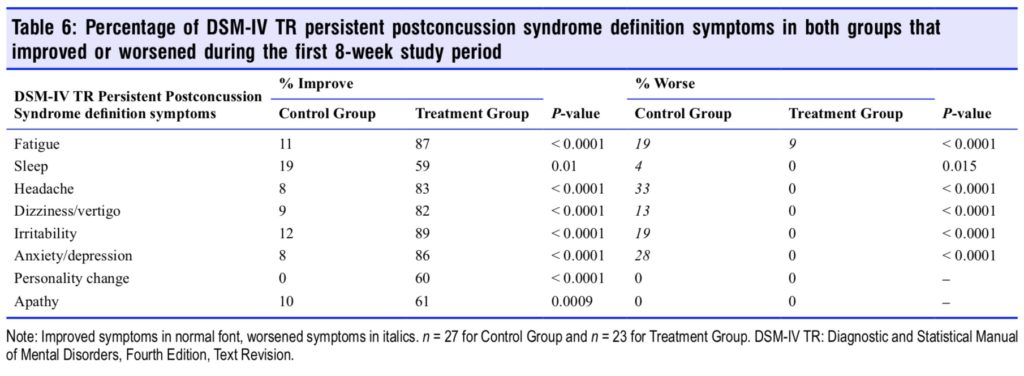
The percentage that each of the PPCS Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV TR) definition symptoms improved or worsened for both groups during the 8-week HBOT and con- trol period are shown in Table 6. Treatment Group subjects experienced significant improvement in all eight of the PPCS definition symptoms; however, easy fatigability, headache, vertigo/dizziness, irritability, and anxiety/depression were the most responsive symptoms to HBOT. The Control Group experienced worsening on six of eight symptoms during the control period.
Both groups completed the HBOT treatment periods in near-identical times: 57.0 ± 5.02 days for the Control Group, 56.5 ± 5.00 days for the Treatment Group (P = 0.7144). The planned 2-month follow-up testing occurred in 79 days for the Treatment Group and 80 days for the Control Group, over 11 weeks for both groups. Eighty-seven percent of subjects were able to complete 40 HBOTs in 8 weeks and 96% were able to complete at least 30 HBOTs. There was no significant difference between Treatment Group (HBOT) and Control Group (control period) in the numbers in each group who experienced either an increase or decrease in psychoactive medication usage; however, a trend favored a reduction in the Treatment Group (P = 0.0785). Both groups reduced psychoactive medication usage by 30–41% during HBOT, but the difference between groups was insignificant (P = 0.4492). There was no difference between civilian and military subjects in PPCS and PTSD symptom reduction after HBOT (P = 0.2320 NSI, P = 0.3818 PCL).
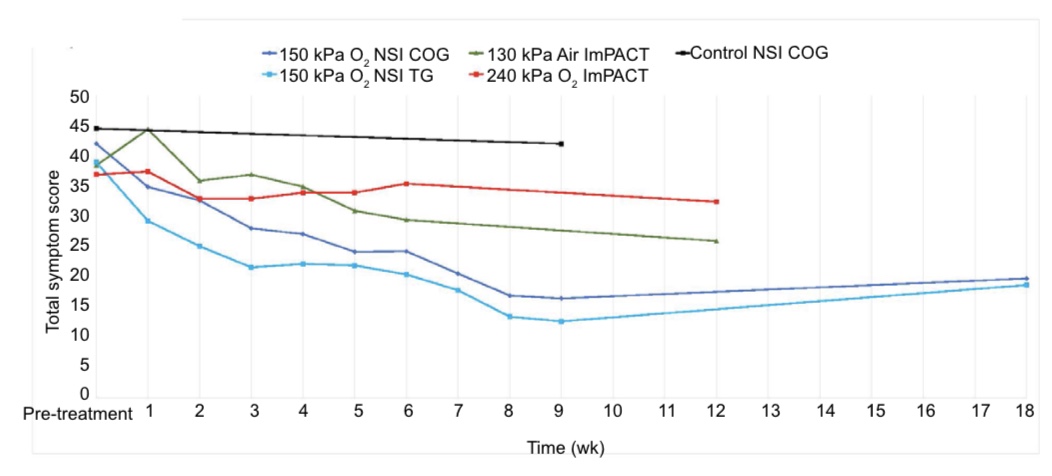
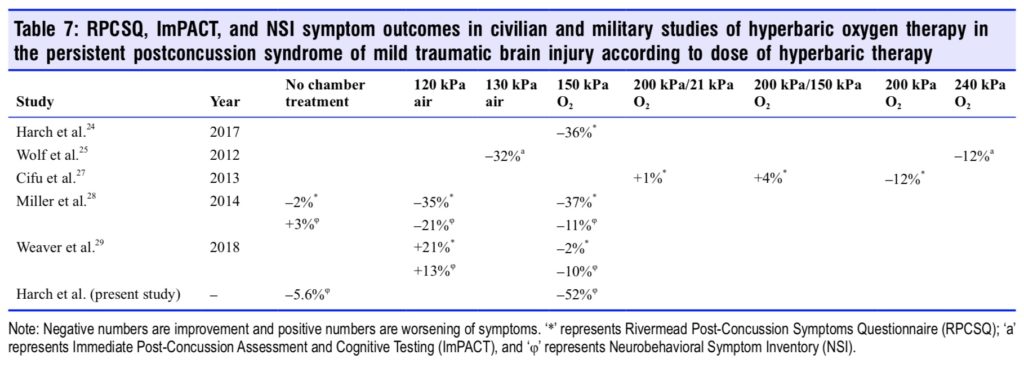
Trajectory of weekly NSI scores during HBOT treatment for both groups and during the control period for Control Group are plotted in Figure 4 (data in Additional Table 1) along with corresponding trajectories of extracted Immediate Postconcussion Assessment and Cognitive Testing (ImPACT) symptom scores for the 240 kPa oxygen and 130 kPa air groups from Wolf et al.25 The trajectories for the Control Group and Treatment Group during HBOT are near identical, but dif- ferent from the Wolf et al.25 groups and the Control Group in the control period. Comparison of ours and the Wolf et al.25 symptom scores to symptom scores in all other studies of HBOT in mTBI/PPCS are shown in Table 7.
Figure 4: Symptom trajectories of total persistent postconcussion syndrome symptom scores during and post-treatment or control.
Note: NSI: Neurobehavioral Symptom Inventory; ImPACT: Immediate Post-Concussion Assessment and Cognitive Testing; COG: Control Group; TG: Treatment Group. ImPACT data were from Wolf et al.25
Complications/Side-Effects
One Serious Adverse Event, a psychiatric deterioration/hospitalization which occurred 1 week after completion of HBOT was an annual Fall occurrence for a military subject that was deemed unrelated to HBOT. Two Unexpected Adverse Events/ Unexpected Suspected Adverse Reactions occurred in two subjects who experienced fatigue with a reversal of improved symptoms late in the HBOT protocol (39 and 34 HBOTs). This was attributed to oxidative stress/overdosing that resolved after 10 days and 4 weeks, respectively. All three events were reported to the Institutional Review Boards and U.S. Food and Drug Administration in Safety Reports. Mild reversible middle ear barotrauma during the prodrome of an upper respiratory infection occurred in one subject and perforation of a multiply previously perforated tympanic membrane (an expected and informed risk for this subject) in another subject during her first HBOT. She finished her HBOT course. Overall, there was an 8% (4/50 subjects) complication rate that was related to the HBOT.
Discussion
This randomized clinical trial was undertaken to con- firm22,24,26,28,29,39 or refute25,27 the efficacy of the 150 kPa oxygen dose of HBOT in mTBI PPCS. This study confirmed the ef- ficacy of 150 kPa HBOT by demonstrating statistically and clinically significant, multi-domain improvements in patients with the PPCS of mTBI 4.6 years after their last TBI. This is the longest average delay to treatment of any of the mTBI/ PPCS HBOT studies published.
Important findings in this study include significant improvements in postconcussion symptoms and seven other outcome variables [memory, cognition/speed of information processing (a computerized cognitive test battery, ANAM, developed and employed by the U.S. military for TBI), depression, anxiety, PTSD symptoms, sleep, and quality of life] in PPCS sub- jects treated with HBOT compared to a randomly assigned Control Group during the same period. The Control Group subsequently experienced the near identical and statistically indistinguishable improvements as the Treatment Group when they were crossed over and received HBOT. The improvement in PPCS symptoms (NSI) cannot be explained by test-retest improvements which have been shown to be minimal in a 30-day period or longer88 and less than the significant reliable change of eight points.88 Our subjects experienced a 26.3-point reduction in the NSI.
The NSI symptom improvement was mirrored in the improvements in DSM-IV TR PPCS definition54 symptoms. All eight DSM-IV TR PPCS symptoms were highly significantly improved in the Treatment Group compared to the Control Group while 13–38% of the Control Group demonstrated worsening of five of the eight symptoms during the control period. The only symptom that worsened for the Treatment Group was fatigue; 9% reported increased fatigue. This may have been a sign of oxidative stress which appeared to be clinically significant in 4/50 subjects late in the protocol. This phenomenon was previously reported in a chronic brain injury HBOT study that employed higher doses or longer courses of HBOT 89 and was possibly responsible for the “trend toward harm” in the 240 kPa oxygen group of Wolf et al.25 as reported by Scorza et al.90 The improvements in the NSI and DSM-IV TR PPCS definition symptoms are the dominant findings in this study. Since symptoms are the primary target of treatment in PPCS91 these findings have the greatest implications for patients with PPCS.
The results of the study are buttressed by multiple factors: 1) improvement in headache; 2) the use of a randomly assigned Control Group; 3) significant improvement in seven other outcome variables despite overall small sample size (n = 50) and smaller n of the Treatment Group compared to the Control Group (23 vs. 27); and 4) improvements post-HBOT with continued improvements in the nearly 3-month followup period that are generally contrary to the natural history of mTBI PPCS and uncharacteristic of placebo effects. The index inclusion criteria symptom for this study (headache) showed improvement in 83% of the Treatment Group, similar to 93% of military subjects with headache in another study on mTBI PPCS with PTSD. 24 During the same period 33% of Control Group experienced worsening of headaches. This symptom has been identified as a primary symptom in TBI,57-59,91 the sole symptom distinguishing TBI/PPCS from PTSD,57 and is a surrogate marker for brain wounding in mTBI.10,92-95 The reduction in headache underscored that HBOT was treating TBI in this study and not just symptoms.91
The randomized controlled single-blinded design of the study was chosen to eliminate multiple causes of possible confounding and demonstrated that HBOT was responsible for the changes and improvements in symptoms, cognitive function, and emotional status as opposed to placebo effects or test-retest effects. This conclusion was supported by the data in Harch et al.23,24 where the magnitude of improvement was similar to our study, but the magnitude of those improvements was criticized because of the presence of PTSD and the lack of a treatment control.96 The present study excluded clinical PTSD, had a far lower PCL score (38.9 vs. 63.4 in Harch et al.24) and a treatment Control Group, yet the HBOT group in our study still showed significant cognitive and affective improvements compared to the Control Group. The conclusions of our study are further supported by the significant functional imaging findings in both Harch et al.23,24 (military subjects) and Boussi-Gross et al.26 (civilian subjects) which were associated with significant improvement in symptoms, cognition, and emotional status similar to our study. Both stud- ies demonstrated global improvements in brain blood flow and the Harch et al.24 study showed a normalization of pattern of blood flow that “could not be explained by placebo effects.”23,24
Significant improvements occurred in the Treatment Group in the other seven outcome variables, including Memory Index and ANAM, compared to Control Group during the control period despite overall small sample size of the study (50 subjects) and disproportionately smaller sample size for the Treatment Group (23 vs. 27). In addition, the Treatment Group experienced non-significant increases in working memory, information processing speed, executive function, and Full Scale Intelligence Quotient (FSIQ) compared to the Control Group. The inability to achieve statistical significance for these 5 cognitive domains may be due to ineffectiveness of HBOT in these domains, test-retest effects, small sample size of the study and disproportionate smaller sample size in the Treatment Group than the Control Group, and the effects of 1.6 years of additional education in the Control Group on these cognitive domains.
The post-HBOT improvements in 11 and 13 outcomes seen in the Treatment Group and Control Group immediately after HBOT and continued improvements in memory, working memory, FSIQ, and processing speed in the nearly 3 months after HBOT (a possible tail-effect) are contrary to the natural history of mTBI PPCS, suggesting a cause and effect relationship of HBOT on improvement of PPCS deficits. The Treatment Group showed 58%, 76%, and 20% change score increases in Memory Index, FSIQ, and processing speed in the nearly 3-month follow-up period while the Control Group demonstrated 41%, 46%, and 37% increases, respectively. The natural history of PPCS as documented by the Veterans Administration,7 Defense and Veterans Brain Injury Center,97 and a civilian study 6 showed a continued requirement for care or persistence of TBI symptoms for 4 years, 1 year, and 3 years, respectively. Post-HBOT further cognitive and affective improvements were demonstrated for symptoms in Harch et al.24 6 months after HBOT and in Wolf et al.25 6 weeks after treatment. They were not demonstrated in Weaver et al.29 for either symptoms or cognition where the 150 kPa HBOT group gains compared to the purported sham group were diminished by 3 months follow-up. The Weaver et al.29 results may be explained by the 70% of subjects with high risk for sleep apnea98; cumulative effects of untreated sleep apnea may have eroded the improvements seen with HBOT in 3 months following HBOT. In addition, negative effects of testing at altitude in Colorado Springs (> 6000 feet, < 81 kPa) post-receiving HBOT at sealevel in two of three sites may have had a deleterious effect on performance similar to what was demonstrated in asymptomatic college students with remote mTBI with loss of consciousness99 and an animal model of HBOT in chronic mTBI.21 Pending medical boarding or dis- ability status/compensation may have also influenced Weaver et al.’s29 results. The tail-effects observed in our study, Harch et al.24 and Wolf et al.25 are consistent with and possibly ex- plained by HBOT’s gene expression100-104 trophic changes105-111 that appear to be progressive.
The cognitive data reinforced a finding in Harch et al.,24 where subjects stated that they were abnormal/different from their premorbid level of function, yet most of their scores at time of randomization were in the normal range. After HBOT patients expressed that they felt more back to normal as in Harch et al.,24 were symptomatically and cognitively improved, and their scores were statistically and clinically improved. This indicated that they in fact were not at their “normal” level of function after their TBI even though their scores were in the “normal” range on standardized testing. Working memory was 96.3 and 104 pre-HBOT in Harch et al.24 and in this study and improved to 107.6 (+11.3 points) in Harch et al.24 and 113.7 (+10.2 points-Treatment Group) and 118.6 (+14 points- Control Group) in this study after HBOT. These “normal” WM scores suggest that reliance on a statistical deficit in memory compared to normals for the DSM-IV TR definition of PPCS may be insensitive when diagnosing PPCS. The common as- sumptions that mTBI does not affect IQ and that a “normal” FSIQ excludes mTBI cognitive deficits96,112,113 appear to be erroneous as well. In both Harch et al.24 and this study the pre-HBOT FSIQs were normal (98 in Harch et al.24 and 106 herein) and yet the subjects had mTBI and cognitive deficits. After HBOT the FSIQ improved 14.2 points in Harch et al.24 and 11.6 (Treatment Group) and 13.4 points (Control Group) in the current study, nearly a standard deviation.
Multiple researchers11,12,24,42-46 have pointed out that the differences in data and conclusions of all of the mTBI PPCS HBOT studies22-29,39,114,115 are best explained by different effects/ outcomes of different doses of hyperoxia and/or hydrostatic pressure, including the most recent study by Weaver et al.29 The cluster of U.S. Department of Defense-sponsored studies characterized different doses of hyperbaric therapy as sham controls. The sham groups, according to the definition of sham51 and the known bioactivity of hydrostatic pressure,50 were actually alternate doses of hyperbaric therapy.11,12,24 The mischaracterization of the low-pressure air doses as sham is supported by the headache data and the symptom trajectories during HBOT. Wolf et al.25 reported a significant (P = 0.002) 41% reduction in mean headache score on the ImPACT with the 130 kPa hyperbaric air group, but a non-significant 21% reduction in the 240 kPa oxygen group, while Cifu et al.27 reported no significant reduction in headache (Item 3) on the Rivermead post-concussion symptoms questionnaire with three different doses of HBOT and Harch et al.24 noted a 93% reduction and an 88% decrease in the current study. The other U.S. Department of Defense studies28,29 did not report head- ache. The trajectory symptom data in Figure 4 shows different symptom trajectories for the NSI for the 150 kPa oxygen and Control Groups in the current study and the ImPACT 240 kPa oxygen and 130 kPa air doses in Wolf et al.25 All three trajectories are typical drug treatment response patterns that are distinctly different from placebo effect patterns identified in pharmaceutical studies.116 More importantly, the 240 kPa oxygen dose suggests a drug toxicity effect24 (improvement then loss of improvement with continued treatment) that was consistent with a “trend toward harm”90 in the isolated mTBI 240 kPa oxygen-treated group in Wolf et al.25 The differences in headache reduction and symptom trajectories in these stud- ies suggest the differing effects of different doses of HBOT on PPCS11,12,24,26,42,43 and are inconsistent with placebo25,27,114,115 or ritual effects28 which would have demonstrated similar effects across all studies.
The finding from all of the HBOT-treated mTBI/PPCS studies is that two doses of hyperbaric therapy have shown benefit (150 kPa oxygen and 130 kPa air), three doses have shown no benefit (200 kPa pressure with three different doses of oxygen), one dose has shown equivocal results (120 kPa air), and one dose (240 kPa oxygen) is potentially harmful.90 Consistent with U.S. Food And Drug Administration Investiga- tional New Drug evaluations this cluster of studies represents a dose-response evaluation of the dual components of HBOT, pressure and hyperoxia, in mTBI PPCS. The consistent finding is that all studies on HBOT in mTBI PPCS,22,24,26,28,29 including the current study, that have used the 150 kPa oxygen dose first pioneered in acute severe TBI,117 used in chronic TBI,19,20,30-38 and confirmed in an animal model of chronic mild TBI,21 have shown statistically significant improvement in subjects. It is apparent that 40 treatments of 150 kPa oxygen for 60 minutes in an eight to ten-week period is a beneficial, valid, and durable treatment for mTBI PPCS. In addition, given the evidence for brain wounding in mTBI PPCS,10,92,93,95 HBOT’s known effects on wound-healing14 and reparative/trophic effects in chronic animal mTBI21 and human mTBI PPCS,24,26,111 HBOT may be the first disease-modifying therapy91 for mTBI PPCS.
Limitations of the study
The crossover design is a minor limitation in that it precluded characterization of a post-control longitudinal comparison to the Treatment Group. Since the natural history of mTBI PPCS is well known to be permanent after a period of time, however, no spontaneous improvement post-control period would be expected. The absence of a non-crossover 2-month Control Group follow-up period does not weaken the conclusions of the study. A second limitation was lack of blinding of subjects to allocation. This was unavoidable since no true pressure control group methodology has been identified in hyperbaric therapy; however, the potential placebo effects of chamber experience and “ritual” have been seriously questioned.24 A third limitation is non-blinding of subjects to the principal investigator, the frequent interaction with the principal investigator during HBOT, and the non-blinded administration of the NSI by the hyperbaric technician at the treatment site. These factors likely contributed to the substantial treatment effect demonstrated for the NSI, but it does not explain the significant improvements in the other outcome instruments compared to the Control Group which were administered by the blinded neuropsychologist. A final limitation was the number of dropouts which necessitated increasing the sample size of the study.
Conclusions
A course of 40 daily, 5 days/week, 150 kPa 60-minute HBOT treatments delivered to civilian and military subjects with the persistent postconcussion syndrome of mild TBI an average of 4.6 years after last TBI resulted in significant improvements in postconcussion symptoms, cognitive variables (memory,
cognition/speed of information processing), and behavioral/ emotional problems (anxiety, depression, PTSD symptoms, sleep, and quality of life) compared to a randomly assigned Control Group. These improvements were duplicated in the Control Group after crossing over to HBOT. In both groups most of the improvements were sustained and even improved for some tests nearly 3 months after the last HBOT, suggest- ing HBOT as a disease-modifying therapy for mTBI PPCS.
Acknowledgements
The investigators wish to thank Christine Watters, PhD, for her work on the statistical analysis of the data and the tax-paying and voting citi- zens of the United States, former Honorable Rep. Rodney Alexander (R, House of Representatives, Louisiana), former Louisiana Senators David Vitter and Mary Landrieu, former congressional appropriator/ legislative aide William A. Duncan, PhD, and former Sec. of the Army Honorable Martin Hoffman (deceased) for the congressional appropriation that made this study a reality. It was primarily funded by Award W81XWH-10-1-0962, $1.2 million, U.S. Army Medical Research and Materiel Command Fort Detrick, Maryland 21702-5012. In addition, we thank the Joe and Dorothy Dorsett Brown Foundation of Metairie, Louisiana for a $40,000 bridging grant to finish the study and acknowledge the significant contributions of Mercy Medical An- gels and Angel Wings for Veterans, Virginia Beach, Virginia for their gratis transportation of veterans and civilians from the continental U.S. and Canada to participate in this study, 1st Financial Bank USA Dakota Dunes, South Dakota for the support of veterans in the study, the office staff of Paul G. Harch, MD, who assisted in scheduling and logistical details for all of the subjects, and Lydia Brown, Loyola University student, who formatted all of the figures in the study.
Author Contributions
Drs. Harch and Andrews conceived and designed the study, performed literature searches, clinical evaluations, data acquisition and analysis, and prepared, edited, and reviewed the manuscript. Cara Rowe and Johannes Lischka performed clinical evaluations, data acquisition and analysis, and prepared, edited, and reviewed the manuscript. Dr. Mark Townsend helped design the study, performed literature searches, clinical evaluations, and prepared and reviewed the manuscript. Drs. Qingzhao and Mercante helped design the study, performed statistical analyses, and prepared and reviewed the manuscript.
Conflicts of Interest
Dr. Harch owns a small consulting company called Harch Hyper- barics, Inc. He also has a financial arrangement with the treatment facility which is the primary location of his medical practice. Part of the manuscript was presented at Hyperbaric Medicine International: HBOT 2019, The 13th Annual Hyperbaric Medicine Symposium in Charleston, SC, USA.
Financial Support
This study was supported by U.S. Army Medical Research and Ma- teriel Command Fort Detrick, No. W81XWH-10-1-0962, and Joe W. and Dorothy Dorsett Brown Foundation.
institutional review board statement
The study protocol was approved by the Institutional Review Boards of the United States Army Medical Research and Materiel Command Office of Research Protections Human Research Protection Office and the Louisiana State University School of Medicine (approval No. 7381) and registered on ClinicalTrials.gov (NCT02089594) on March 18, 2014.
Informed Consent Statement
The authors certify that they have obtained all appropriate patient consent forms. In the form the patients or their legal guardians have given their consent for patients images and other clinical information to be reported in the journal. The patients or their legal guardians understand that their names and initials will not be published.
Reporting Statement
The writing and editing of the article were performed in accordance with the CONsolidated Standards Of Reporting Trials (CONSORT) Statement.
Copyright Transfer Agreement
The Copyright License Agreement has been signed by all authors before publication.
Data Sharing Statement
Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
Open Access Statement
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial- ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional Files
Additional file 1: Full details of the Methods and Protocol. Additional file 2: Construction of categorical variables to measure cognitive function.
Additional Table 1: Total persistent postconcussion syndrome symp- tom scores this study and Wolf et al.25 during and post-treatment or control.
References
1. Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic brain injuryrelated
emergency department visits, hospitalizations, and deaths
– United States, 2007 and 2013. MMWR Surveill Summ. 2017;66:1-
16.
2. Centers for Disease Control and Prevention. Report to Congress on
mild traumatic brain injury in the United States: steps to prevent a
serious public health problem. Vol 45. Centers for Disease Control
and Prevention: Atlanta, GA, USA. 2003.
3. Zogg CK, Haring RS, Xu L, et al. Patient Presentations in outpatient
settings: epidemiology of adult head trauma treated outside of
hospital emergency departments. Epidemiology. 2018;29:885-894.
4. Zogg CK, Haring RS, Xu L, et al. The Epidemiology of pediatric
head injury treated outside of hospital emergency departments.
Epidemiology. 2018;29:269-279.
5. McInnes K, Friesen CL, MacKenzie DE, Westwood DA, Boe SG.
Mild traumatic brain injury (mTBI) and chronic cognitive impairment:
A scoping review. PLoS One. 2017;12:e0174847.
6. Hiploylee C, Dufort PA, Davis HS, et al. Longitudinal study of
postconcussion syndrome: not everyone recovers. J Neurotrauma.
2017;34:1511-1523.
7. Congress of the United States, Congressional Budget Office. A
CBO study: The Veterans Health Administration’s treatment of
PTSD and traumatic brain injury among recent combat veterans.
http://timemilitaryfileswordpresscom/2012/02/02-09-ptsd-1pd.
Last accessed January 5, 2020.
8. Cooper DB, Bunner AE, Kennedy JE, et al. Treatment of persistent
post-concussive symptoms after mild traumatic brain injury: a
systematic review of cognitive rehabilitation and behavioral health
interventions in military service members and veterans. Brain Imaging
Behav. 2015;9:403-420.
9. Diaz-Arrastia R, Kochanek PM, Bergold P, et al. Pharmacotherapy
of traumatic brain injury: state of the science and the road forward:
report of the Department of Defense Neurotrauma Pharmacology
Workgroup. J Neurotrauma. 2014;31:135-158.
10. Wallace EJ, Mathias JL, Ward L. Diffusion tensor imaging changes
following mild, moderate and severe adult traumatic brain injury: a
meta-analysis. Brain Imaging Behav. 2018;12:1607-1621.
11. Harch PG. Hyperbaric oxygen therapy for post-concussion syndrome:
contradictory conclusions from a study mischaracterized
as sham-controlled. J Neurotrauma. 2013;30:1995-1999.
12. Harch PG. Hyperbaric oxygen in chronic traumatic brain injury:
oxygen, pressure, and gene therapy. Med Gas Res. 2015;5:9.
13. Harch PG. HBO therapy in global cerebral ischemia/anoxia and
coma. Textbook of Hyperbaric Medicine. Cham: Springer International
Publishing; 2017:269-319.
14. Undersea and Hyperbaric Medical Society, Hyperbaric Oxygen
Committee. Hyperbaric Oxygen Therapy indications: The Hyperbaric
Oxygen Therapy Committee Report. Best Publishing Company.
2014.
15. Centers for Medicare and Medicaid Services. National Coverage Determination
(NCD) Hyperbaric Oxygen Therapy (20.29). National
Coverage Determinations (NCD) Manual, Publication Number 100-
3, Chapter 1, Part 1, Section 2029 effective date June 19, 2006. https://
www.cms.gov/medicare-coverage-database/details/ncd-details.aspx
?NCDId=12&ncdver=4&DocID=20.29&ncd_id=20.29&ncd_versio
n=3&basket=ncd*3a%2420.29*3a%243*3a%24Hyperbaric+Oxyge
n+Therapy&bc=gAAAAAgAAAAA&. Last accessed February 23,
2020.
16. Jain KK. Worldwide overview of hyperbaric medicine, Chapter 49.
In: Jain KK, ed. Textbook of Hyperbaric Medicine. 6th ed. Cham:
Springer; 2017:609-614.
17. Mathieu D, Marroni A, Kot J. Tenth European Consensus Conference
on Hyperbaric Medicine: recommendations for accepted and
non-accepted clinical indications and practice of hyperbaric oxygen
treatment. Diving Hyperb Med. 2017;47:24-32.
18. Takahashi H, Yagi H. Hyperbaric Medicine in Japan, Chapter 42.
In: Jain KK, ed. Textbook of Hyperbaric Medicine. 5th ed. Gottingen,
Germany: Hogrefe and Huber Publishers; 2009:495-498.
19. Harch PG. Hyperbaric oxygen therapy in the treatment of chronic
traumatic brain injury: from Louisiana boxers to US veterans, an
American Chronology. Wound Care Hyperbaric Med. 2010;1:26-
34.
20. Harch P, Gottlieb S, Van Meter K, Staab P. HMPAO SPECT brain
imaging and low pressure hbot in the diagnosis and treatment of
chronic traumatic, ischemic, hypoxic and anoxic encephalopathies.
Undersea Hyperb Med. 1994;21:S30.
21. Harch PG, Kriedt C, Van Meter KW, Sutherland RJ. Hyperbaric oxygen
therapy improves spatial learning and memory in a rat model
of chronic traumatic brain injury. Brain Res. 2007;1174:120-129.
22. Harch PG, Fogarty EF, Staab PK, Van Meter K. Low pressure hyperbaric
oxygen therapy and SPECT brain imaging in the treatment
of blast-induced chronic traumatic brain injury (post-concussion
syndrome) and post traumatic stress disorder: a case report.
Cases J. 2009;2:6538-6538.
23. Harch PG, Andrews SR, Fogarty EF, et al. A phase I study of lowpressure
hyperbaric oxygen therapy for blast-induced post-concussion
syndrome and post-traumatic stress disorder. J Neurotrauma.
2012;29:168-185.
24. Harch PG, Andrews SR, Fogarty EF, Lucarini J, Van Meter KW.
Case control study: hyperbaric oxygen treatment of mild traumatic
brain injury persistent post-concussion syndrome and post-traumatic
stress disorder. Med Gas Res. 2017;7:156-174.
25. Wolf G, Cifu D, Baugh L, Carne W, Profenna L. The effect of
hyperbaric oxygen on symptoms after mild traumatic brain injury.
J Neurotrauma. 2012;29:2606-2612.
26. Boussi-Gross R, Golan H, Fishlev G, et al. Hyperbaric oxygen
therapy can improve post concussion syndrome years after mild
traumatic brain injury – randomized prospective trial. PLoS One.
2013;8:e79995.
27. Cifu DX, Hart BB, West SL, Walker W, Carne W. The effect of hyperbaric
oxygen on persistent postconcussion symptoms. J Head
Trauma Rehabil. 2014;29:11-20.
28. Miller RS, Weaver LK, Bahraini N, et al. Effects of hyperbaric
oxygen on symptoms and quality of life among service members
with persistent postconcussion symptoms: a randomized clinical
trial. JAMA Intern Med. 2015;175:43-52.
29. Weaver LK, Wilson SH, Lindblad AS, et al. Hyperbaric oxygen
for post-concussive symptoms in United States military service
members: a randomized clinical trial. Undersea Hyperb Med.
2018;45:129-156.
30. Harch PG, Van Meter KW, Neubauer RA, Gottlieb SF. Use of HMPAO
SPECT for Assessment of Response to HBO in Ischemic/
Hypoxic Encephalopathies, Appendix. In: Jain KK, ed. Textbook
of Hyperbaric Medicine, 2nd ed. Seattle, WA, USA: Hogrefe and
Huber Publishers; 1996:480-491.
31. Harch PG, Neubauer RA. Hyperbaric oxygen therapy in global
cerebral ischemia/anoxia and coma, Chapter 18. In: Jain KK, ed.
Textbook of Hyperbaric Medicine. 3rd Revised Edition. Seattle,
WA, USA: Hogrefe and Huber Publishers; 1999:319-345.
32. Harch PG, Neubauer RA. Hyperbaric oxygen therapy in global
cerebral ischemia/anoxia and coma, Chapter 18. In: Jain KK, ed.
Textbook of Hyperbaric Medicine. 3rd Revised Edition. Seattle,
WA, USA: Hogrefe and Huber Publishers; 2004:223-261.
33. Harch PG, Neubauer RA. Hyperbaric oxygen therapy in global
cerebral ischemia/anoxia and coma, Chapter 19. In: Jain KK, ed.
Textbook of Hyperbaric Medicine. 5th Revised Edition. Seattle,
WA, USA: Hogrefe and Huber Publishers; 2009:235-274.
34. Harch PG, Neubauer RA, Uszler JM, James PB. Appendix: Diagnostic
imaging and HBO therapy, Chapter 44. In: Jain KK, ed.
Textbook of Hyperbaric Medicine. 5th Revised Edition. Seattle,
WA, USA: Hogrefe and Huber Publishers; 2009:505-519.
35. Neubauer RA, Gottlieb SF, Pevsner NH. Hyperbaric oxygen for
treatment of closed head injury. South Med J. 1994;87:933-936.
36. Golden ZL, Neubauer R, Golden CJ, Greene L, Marsh J, Mleko A.
Improvement in cerebral metabolism in chronic brain injury after
hyperbaric oxygen therapy. Int J Neurosci. 2002;112:119-131.
37. Golden Z, Golden CJ, Neubauer RA. Improving neuropsychological
function after chronic brain injury with hyperbaric oxygen.
Disabil Rehabil. 2006;28:1379-1386.
38. Churchill S, Weaver LK, Deru K, et al. A prospective trial of hyperbaric
oxygen for chronic sequelae after brain injury (HYBOBI).
Undersea Hyperb Med. 2013;40:165-193.
39. Wright JK, Zant E, Groom K, Schlegel RE, Gilliland K. Case
report: Treatment of mild traumatic brain injury with hyperbaric
oxygen. Undersea Hyperb Med. 2009;36:391-399.
40. Mozayeni BR, Duncan W, Zant E, Love TL, Beckman RL, Stoller
KP. The National Brain Injury Rescue and Rehabilitation Study –
a multicenter observational study of hyperbaric oxygen for mild
traumatic brain injury with post-concussive symptoms. Med Gas
Res. 2019;9:1-12.
41. Shytle RD, Eve DJ, Kim SH, Spiegel A, Sanberg PR, Borlongan
CV. Retrospective case series of traumatic brain injury and posttraumatic
stress disorder treated with hyperbaric oxygen therapy.
Cell Transplant. 2019;28:885-892.
42. Figueroa XA, Wright JK. Hyperbaric oxygen: B-level evidence
in mild traumatic brain injury clinical trials. Neurology.
2016;87:1400-1406.
43. Hadanny A, Efrati S. Treatment of persistent post-concussion syndrome
due to mild traumatic brain injury: current status and future
directions. Expert Rev Neurother. 2016;16:875-887.
44. Marois P, Mukherjee A, Ballaz L. Hyperbaric oxygen treatment
for persistent postconcussion symptoms–a placebo effect? JAMA
Intern Med. 2015;175:1239-1240.
45. Mychaskiw G 2nd, Stephens PL. Hyperbaric oxygen, mild traumatic
brain injury, and study design: an elusive target. J Neurotrauma.
2013;30:1681-1682.
46. Mychaskiw G. Known knowns, known unknowns and unknown
unknowns: the science and the passion of HBO2 therapy and
traumatic brain injury: an editorial perspective. Undersea Hyperb
Med. 2013;40:371-372.
47. Hu Q, Manaenko A, Guo Z, Huang L, Tang J, Zhang JH. Hyperbaric
oxygen therapy for post concussion symptoms: issues may
affect the results. Med Gas Res. 2015;5:10.
48. Weaver LK, Cifu D, Hart B, Wolf G, Miller S. Hyperbaric oxygen
for post-concussion syndrome: design of Department of Defense
clinical trials. Undersea Hyperb Med. 2012;39:807-814.
49. Mitchell SJ, Bennett MH. Unestablished indications for hyperbaric
oxygen therapy. Diving Hyperb Med. 2014;44:228-234.
50. Macdonald AG, Fraser PJ. The transduction of very small hydrostatic
pressures. Comp Biochem Physiol A Mol Integr Physiol.
1999;122:13-36.
51. Merriam-Webster. Sham. http://wwwmerriam-webstercom/medical/
sham. Last accessed January 5, 2020.
52. Mild Traumatic Brain Injury Committee of the Head Injury Interdisciplinary
Special Interest Group of the American Congress of
Rehabilitation Medicine. Definition of mild traumatic brain injury.
J Head Trauma Rehabil. 1993;8:86-87.
53. Bigler ED. Neuropsychology and clinical neuroscience of persistent
post-concussive syndrome. J Int Neuropsychol Soc. 2008;14:1-
22.
54. Diagnostic and Statistical Manual of Mental Disorders. 4th ed.,
text revised. Washington, D.C.: American Psychiatric Association.
2000.
55. King PR, Donnelly KT, Donnelly JP, et al. Psychometric study
of the neurobehavioral symptom inventory. J Rehabil Res Dev.
2012;49:879-888.
56. Cicerone KD, Kalmar K. Persistent postconcussion syndrome: the
structure of subjective complaints after mild traumatic brain injury.
J Head Trauma Rehabil. 1995;10:1-17.
57. Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA.
Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N
Engl J Med. 2008;358:453-463.
58. Theeler B, Lucas S, Riechers RG 2nd, Ruff RL. Post-traumatic
headaches in civilians and military personnel: a comparative, clinical
review. Headache. 2013;53:881-900.
59. Theeler BJ, Flynn FG, Erickson JC. Chronic daily headache in
U.S. soldiers after concussion. Headache. 2012;52:732-738.
60. Michigan alcohol screening test, Revised. http://counsellingresourcecom/
quizzes/alcoholmast/indexhtml. Last accessed January
5, 2020.
61. Gavin DR, Ross HE, Skinner HA. Diagnostic validity of the drug
abuse screening test in the assessment of DSM-III drug disorders.
Br J Addict. 1989;84:301-307.
62. Post-traumatic stress disorder checklist-military and civilian.
http://wwwptsdvagov/professional/pages/assessments/ptsd-checklist.
asp. Last accessed January 5, 2020.
63. Corrigan JD, Bogner J. Initial reliability and validity of the Ohio
State University TBI Identification Method. J Head Trauma Rehabil.
2007;22:318-329.
64. Blake DD, Weathers FW, Nagy LM, et al. The development of a
Clinician-Administered PTSD Scale. J Traum Stress. 1995;8:75-
90.
65. Tombaugh TN. Test of memory malingering: TOMM. New York:
Multi-Health Systems, Inc. 1996.
66. Lesak M, Howieson D, Loring D. Neuropsychological Assessment.
4th ed. New York: Oxford University Press. 2004:365-367,776.
67. Wechsler D. Wechsler Test of Adult Reading (WTAR)-Manual. San
Antonio, TX, USA: The Psychological Corporation. 2001.
68. Hedlund JL, Vieweg BW. The Hamilton rating scale for depression:
a comprehensive review. J Operat Psychiatry. 1979;10:149-
165.
69. Hamilton M. The assessment of anxiety states by rating. Br J Med
Psychol. 1959;32:50-55.
70. Wechsler adult intelligence scale-IV. http://pearsonassesscom/
HAIWEB/Cultures/en-us/Product Detailhtm?Pid=015-8980-
808&Mode=summary. Last accessed January 5, 2020.
71. Wechsler abbreviated scale of intelligence. http://pearsonassesscom/
haiweb/cultures/enus/productdetail htm?pid = 015-8981-502.
Last accessed January 5, 2020.
72. Wechsler Memory Scale-IV. http://pearsonassesscom/HAIWEB/
Cultures/en-us/Productdetailhtm?Pid=8895-800. Last accessed
January 5, 2020.
73. Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment.
4 ed. New York: Oxford University Press. 2004:422-429.
74. Sivan AB. Benton Visual Retention Test. 5th ed. San Antonio: The
Psychological Corporation. 1992.
75. Golden C. Stroop color and word test. https://wwwparinccom/
products/pkey/435. Last accessed January 5, 2020.
76. Spreen O, Strauss E. A compendium of neuropsychological tests:
Administration, norms and commentary. 2nd ed. New York: Oxford
University Press. 1998.
77. Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment,
Fourth Edition. New York: Oxford University Press.
2004:520-521.
78. Vincent AS, Roebuck-Spencer TM, Cox-Fuenzalida LE, Block C,
Scott JG, Kane R. Validation of ANAM for cognitive screening in
a mixed clinical sample. Appl Neuropsychol Adult. 2018;25:366-
375.
79. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The
Pittsburgh sleep quality index: A new instrument for psychiatric
practice and research. Psychiatry Res. 1989;28:193-213.
80. Quality of Life After Brain Injury (QOLIBRI). https://wwwqolibrinetcom.
Last accessed January 5, 2020.
81. Heaton R, Miller SW, Taylor MJ, Grant-Isibor I. Revised comprehensive
norms for an expanded Halstead-Reitan Battery: Demographically
adjusted neuropsychological norms for African
American and Caucasian adults. Lutz, FL, USA: Psychological
Assessment Resources. 2004.
82. Soble JR, Silva MA, Vanderploeg RD, et al. Normative Data for
the Neurobehavioral Symptom Inventory (NSI) and post-concussion
symptom profiles among TBI, PTSD, and nonclinical samples.
Clin Neuropsychol. 2014;28:614-632.
83. Wilde EA, Whiteneck GG, Bogner J, et al. Recommendations for
the use of common outcome measures in traumatic brain injury
research. Arch Phys Med Rehabil. 2010;91:1650-1660.e17.
84. Dretsch M, Bleiberg J, Williams K, et al. Three scoring approaches
to the neurobehavioral symptom inventory for measuring clinical
change in service members receiving intensive treatment for
combat-related mTBI. J Head Trauma Rehabil. 2016;31:23-29.
85. McAllister TW. Neurobiological consequences of traumatic brain
injury. Dialogues Clin Neurosci. 2011;13:287-300.
86. Basso MR, Carona FD, Lowery N, Axelrod BN. Practice effects on
the WAIS-III across 3- and 6-month intervals. Clin Neuropsychol.
2002;16:57-63.
87. Tukey JW. Comparing individual means in the analysis of variance.
Biometrics. 1949;5:99-114.
88. Belanger HG, Lange RT, Bailie J, et al. Interpreting change on the
neurobehavioral symptom inventory and the PTSD checklist in
military personnel. Clin Neuropsychol. 2016;30:1063-1073.
89. Harch PG. The dosage of hyperbaric oxygen in chronic brain injury.
In: Joiner JT, ed. The Proceedings of the 2nd International
Symposium on Hyperbaric Oxygenation for Cerebral palsy and
the Brain-Injured Child. Flagstaff, AZ, USA: Best Publishing Co.
2002:31-56.
90. Scorza K, McCarthy W, Miller R, Carne W, Wolf G. Hyperbaric
oxygen effects on PTSD and mTBI symptoms: a subset analysis.
Orlando, FL, USA: Undersea and Hyperbaric Medical Society Annual
Meeting. 2013.
91. Polinder S, Cnossen MC, Real RGL, et al. A multidimensional approach
to post-concussion symptoms in mild traumatic brain injury.
Front Neurol. 2018;9:1113-1113.
92. Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA,
Little DM. White matter integrity and cognition in chronic traumatic
brain injury: a diffusion tensor imaging study. Brain.
2007;130:2508-2519.
93. Lipton ML, Gulko E, Zimmerman ME, et al. Diffusion-tensor
imaging implicates prefrontal axonal injury in executive function
impairment following very mild traumatic brain injury. Radiology.
2009;252:816-824.
94. Roth TL, Nayak D, Atanasijevic T, Koretsky AP, Latour LL, Mc-
Gavern DB. Transcranial amelioration of inflammation and cell
death after brain injury. Nature. 2014;505:223-228.
95. Korn A, Golan H, Melamed I, Pascual-Marqui R, Friedman A.
Focal cortical dysfunction and blood-brain barrier disruption in
patients with Postconcussion syndrome. J Clin Neurophysiol.
2005;22:1-9.
96. Wortzel HS, Arciniegas DB, Anderson CA, Vanderploeg RD,
Brenner LA. A phase I study of low-pressure hyperbaric oxygen
therapy for blast-induced post-concussion syndrome and posttraumatic
stress disorder: a neuropsychiatric perspective. J Neurotrauma.
2012;29:2421-2430.
97. Ferdosi H, Schwab KA, Metti A, et al. Trajectory of postconcussive
symptoms 12 months after deployment in soldiers with and
without mild traumatic brain injury: warrior strong study. Am J
Epidemiol. 2019;188:77-86.
98. Walker JM, Mulatya C, Hebert D, Wilson SH, Lindblad AS, Weaver
LK. Sleep assessment in a randomized trial of hyperbaric oxygen
in U.S. service members with post concussive mild traumatic
brain injury compared to normal controls. Sleep Med. 2018;51:66-
79.
99. Ewing R, McCarthy D, Gronwall D, Wrightson P. Persisting effects
of minor head injury observable during hypoxic stress. J Clin
Exp Neuropsych. 1980;2:147-155.
100. Siddiqui A, Davidson JD, Mustoe TA. Ischemic tissue oxygen
capacitance after hyperbaric oxygen therapy: a new physiologic
concept. Plast Reconstr Surg. 1997;99:148-155.
101. Godman CA, Chheda KP, Hightower LE, Perdrizet G, Shin DG,
Giardina C. Hyperbaric oxygen induces a cytoprotective and angiogenic
response in human microvascular endothelial cells. Cell
Stress Chaperones. 2010;15:431-442.
102. Chen Y, Nadi NS, Chavko M, Auker CR, McCarron RM. Microarray
analysis of gene expression in rat cortical neurons exposed to
hyperbaric air and oxygen. Neurochem Res. 2009;34:1047-1056.
103. Oh S, Lee E, Lee J, Lim Y, Kim J, Woo S. Comparison of the effects
of 40% oxygen and two atmospheric absolute air pressure
conditions on stress-induced premature senescence of normal human
diploid fibroblasts. Cell Stress Chaperones. 2008;13:447-458.
104. Kendall AC, Whatmore JL, Harries LW, Winyard PG, Eggleton P,
Smerdon GR. Different oxygen treatment pressures alter inflammatory
gene expression in human endothelial cells. Undersea Hyperb
Med. 2013;40:115-123.
105. Marx RE, Johnson RP. Problem wounds in oral and maxillofacial
surgery: the role of hyperbaric oxygen, Chapter 4. In: Davis JC,
Hunt TK, eds. Problem Wounds: The Role of Oxygen. New York:
Elsevier Science Publishing Co. 1988:65-123.
106. Brismar K, Lind F, Kratz G. Dose-dependent hyperbaric oxygen
stimulation of human fibroblast proliferation. Wound Repair Regen.
1997;5:147-150.
107. Marx RE, Ehler WJ, Tayapongsak P, Pierce LW. Relationship of
oxygen dose to angiogenesis induction in irradiated tissue. Am J
Surg. 1990;160:519-524.
108. Manson PN, Im MJ, Myers RAM, Hoopes JE. Improved capillaries
by hyperbaric-oxygen in skin flaps. Surg Forum. 1980;31:564-
566.
109. Uhl E, Sirsjö A, Haapaniemi T, Nilsson G, Nylander G. Hyperbaric
oxygen improves wound healing in normal and ischemic skin tissue.
Plast Reconstr Surg. 1994;93:835-841.
110. Ueng SW, Lee SS, Lin SS, et al. Bone healing of tibial lengthening
is enhanced by hyperbaric oxygen therapy: a study of bone mineral
density and torsional strength on rabbits. J Trauma. 1998;44:676-
681.
111. Tal S, Hadanny A, Sasson E, Suzin G, Efrati S. Hyperbaric oxygen
therapy can induce angiogenesis and regeneration of nerve fibers in
traumatic brain injury patients. Front Hum Neurosci. 2017;11:508.
112. Belanger HG, Vanderploeg RD. The neuropsychological impact
of sports-related concussion: a meta-analysis. J Int Neuropsychol
Soc. 2005;11:345-357.
113. Belanger HG, Curtiss G, Demery JA, Lebowitz BK, Vanderploeg
RD. Factors moderating neuropsychological outcomes following
mild traumatic brain injury: a meta-analysis. J Int Neuropsychol
Soc. 2005;11:215-227.
114. Walker WC, Franke LM, Cifu DX, Hart BB. Randomized, shamcontrolled,
feasibility trial of hyperbaric oxygen for service members
with postconcussion syndrome: cognitive and psychomotor
outcomes 1 week postintervention. Neurorehab Neural Repair.
2014;28:420-432.
115. Cifu DX, Walker WC, West SL, et al. Hyperbaric oxygen for blastrelated
postconcussion syndrome: three-month outcomes. Ann
Neurol. 2014;75:277-286.
116. Quitkin FM. Placebos, drug effects, and study design: a clinician’s
guide. Am J Psychiatry. 1999;156:829-836.
117. Holbach KH, Caroli A, Wassmann H. Cerebral energy metabolism
in patients with brain lesions of normo- and hyperbaric oxygen
pressures. J Neurol. 1977;217:17-30.
Received: November 12, 2019
Reviewed: November 19, 2019
Accepted: December 16, 2019