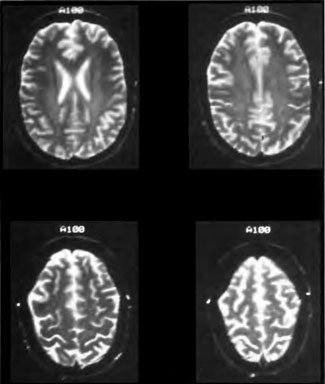
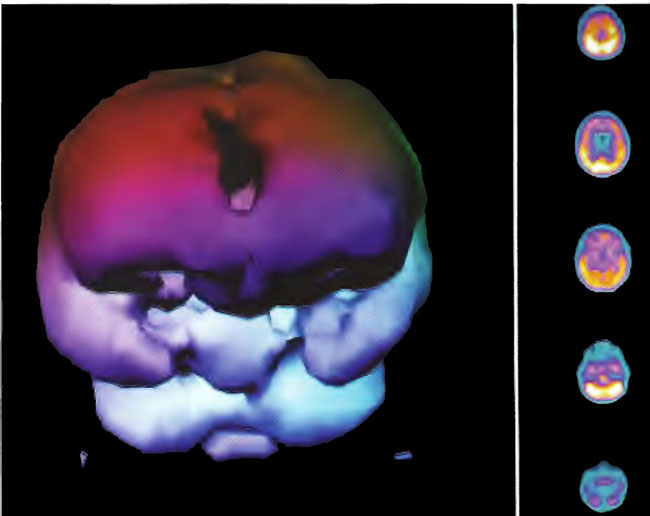
Soon, the first phase of HOTFAST, a study of Hyperbaric Oxygen Therapy for Acute Stroke, will begin. Dr. James F. Toole, Director of the National Stroke Research Center, President of the World Federation of Neurology, and chaired professor of neurology at Bowman Gray/Wake Forest University School of Medicine in Winston-Salem, North Carolina, will be co -principal investigator along with the author. Dr. Richard Neubauer is senior honorary consultant and the main initiative behind the origination of the study.
This study will be conducted in three phases. The first phase is a feasibility study that will entail entering a minimum total of 13 patients at all of the participating centers to assess the ‘feasibility of completing the protocol as well as the safety issues involved. It will be an uncontrolled trial. The data from this study will be submitted to the NIH for the second phase, which would be a fully funded, randomized, prospective,
controlled-blinded trial. Should this second phase show positive results, it may stand alone or necessitate the third phase, a much larger multicenter trial.
This process began in February 1997, when Drs. Neubauer and Toole held an organizing meeting for the trial at the American Academy of Neurology meeting in Boston. Approximately 15 physicians, including neurologists, hyperbaric, and emergency physicians convened to plan the process and get the ball rolling. This spawned a $50,000 gift from Edgar Otto of National Healthnet to hold the Graylyn Conference at Bowman Gray in November 1997. We invited 50 internationally respected stroke, neurology, emergency, and hyperbaric specialists to the meeting. After the meeting I was asked to be the national coordinator and principal investigator. Dr. Toole subsequently joined me and we proceeded with Dr. Neubauer to the Cerebral Congress on Vascular Disease, Dementia, and Aging in Washington, D.C., in May 1998, where we held an open symposium on developing a stroke/HBO protocol.
Prior to this meeting, I performed a personal phone survey of 60 of the most prominent and experienced hyperbaric centers in the U.S. to assess interest in this project. The response was overwhelmingly positive. We put together a team of individuals to work on the project, formed executive and working committees, and recruited nationally respected stroke and hyperbaric experts. Since that time, we have developed the final protocol and screened and selected pilot sites. Initially, we desired five pilot sites, but as the project evolved, the number has grown to 14, with a waiting list of interested centers. We just finished the final meeting of the pilot sites in New Orleans on February 11, 2000, at the American Heart Association’s annual Stroke Meeting. In attendance were approximately 20 individuals, including a member of the NIH Stroke Division.
I am now performing the final tweaking of the protocol and consent forms, which will be mailed to the pilot sites to complete applications to their local Institutional Review Boards. The protocol is for acute stroke, less than or equal to 6 hours old, and the patients will run a five-day inpatient protocol. Neurological outcome will be documented, but is somewhat unimportant for this phase since we are trying to demonstrate only feasibility and safety. Patients will present for a three-month follow-up. SPECT brain imaging is a part of the study at institutions that have the capability.
There is one international site in Germany that originally was to be a part of the study, but they have decided on a different protocol / dose and will proceed on their own in parallel to our study. The first phase is closed at this point, but interested parties can possibly participate in the second and/or third phases that will involve 30 or more centers. The mix of hospitals includes university, county, city, and private for- and not-for-profit. The first phase is not funded. Should we be denied funding by the NIH, we will seek funding from alternate sources.
We are sorely in need of administrative funding to help the project along, since Dr. Toole, our coordinators Dr. George and Virginia Howard, and I are and have been working on the study gratis for over three years. If you are aware of any benefactors who are interested in funding a worthy cause in a tax-deductible manner, please have them contact us. For more information, I can be reached at [email protected] Phone: 504-309-4948, or 504-304-5877; Virginia Howard can be reached at the coordination center at the University of Alabama, Birmingham, 205-934-7197; or Dr. Toole at the National Stroke Research Center, 336-716-2338.