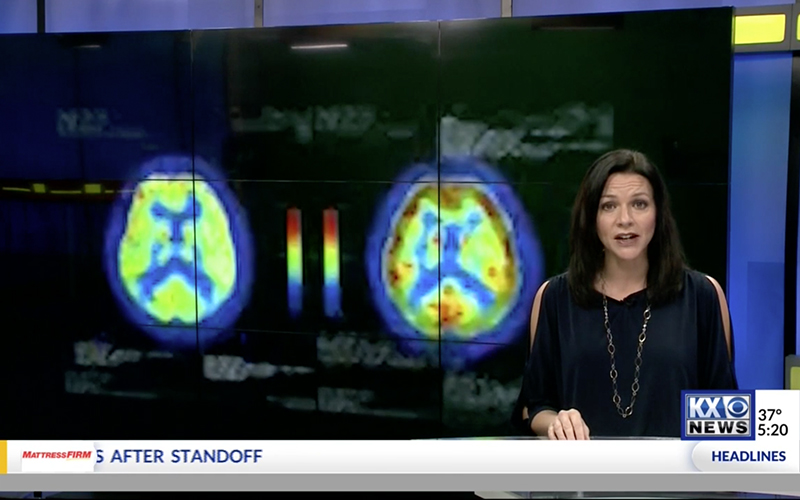
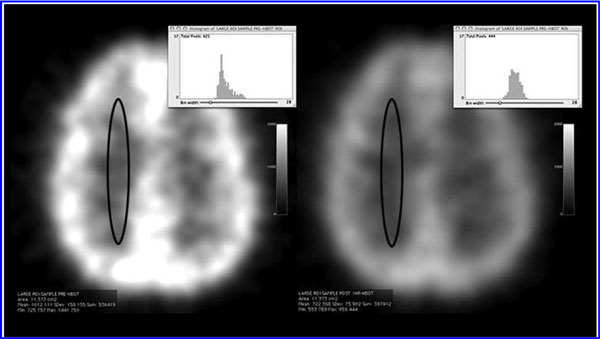
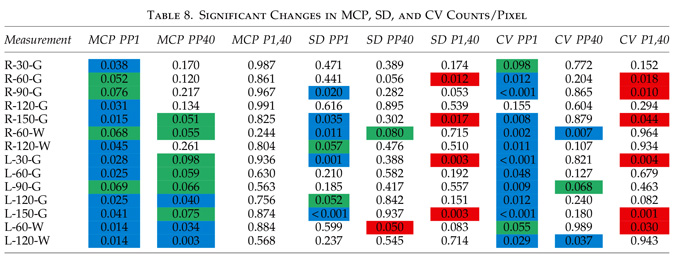
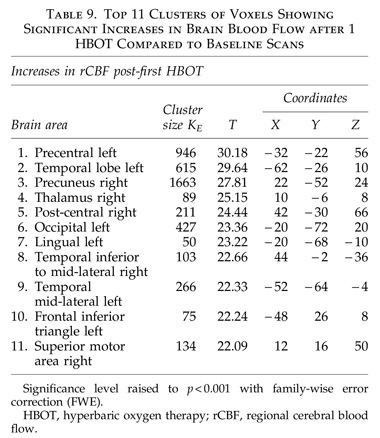
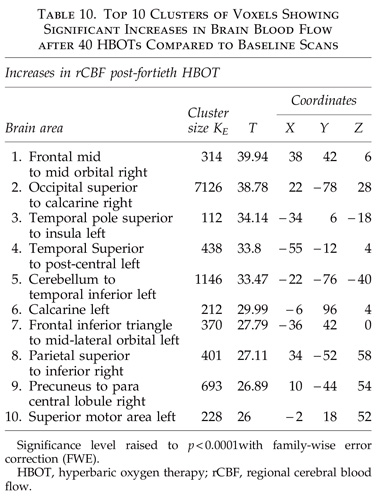
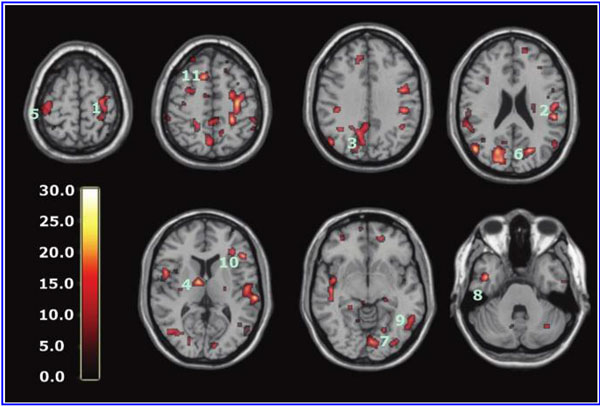
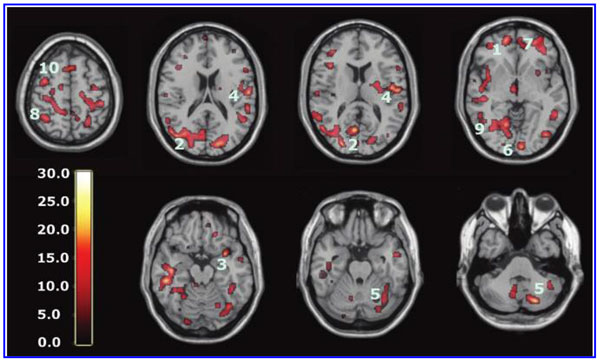
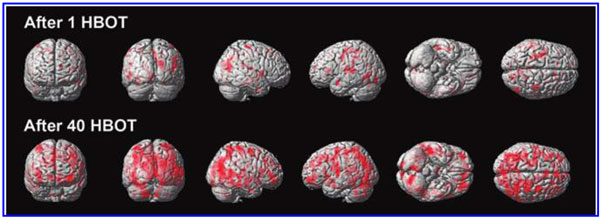
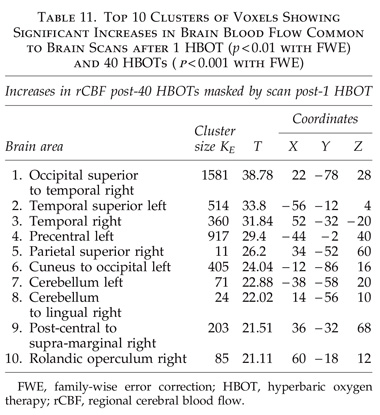
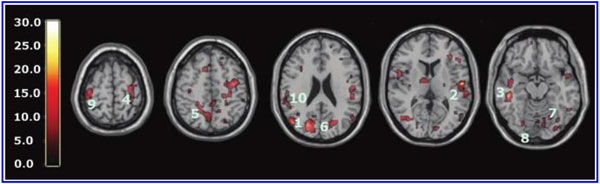
The global improvements in brain blood flow after 1 HBOT in our subjects were associated with improved function after 40 HBOTs, thus supporting the Neubauer effect’s prediction of neurological improvement. SPM analysis demonstrated considerable overlap of the areas with improved blood flow after 1 HBOT with those after 40 HBOTs, indicating that the areas identified on SPECT by the Neubauer effect are likely those responsible for neurological improvement after 40 HBOTs. We have demonstrated the Neubauer effect in severe chronic TBI patients (Harch and Neubauer, 1999, 2004a, 2009b, 2009c; Harch et al., 1994,1996a; Neubauer et al., 1994), along with a pattern shift on SPECT after the first HBOT. The pattern shift consists of normalization (a relative decrease in high and increase in low blood flow; Harch and Neubauer, 1999,2004b; Harch, et al., 1996a) that is captured by a reduction in SD and CV in this study. The first HBOT would not be expected to improve function, however, likely due to the limited impact of a single HBOT on blast-induced degenerated white matter (Bauman et al., 2009).
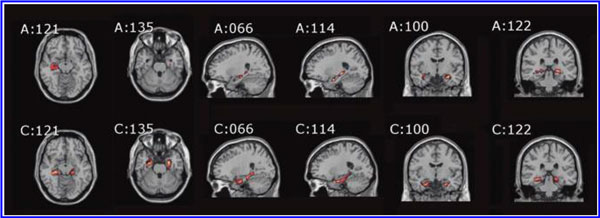
The increased blood flow on SPECT, variance in MCP change, and improved neurological function seen after 40 HBOT sessions suggests a set of mechanisms different from those that occur after 1 HBOT session. We propose that these mechanisms are the typical trophic mechanisms of HBOT in chronic non-central nervous system wounds (Gesell, 2009). Repetitive HBOT stimulates angiogenesis in chronic non-CNS wounded tissue (Marx et al., 1990), most likely by genomic effects (Godman et al., 2009), and has been shown to increase blood vessel density in injured hippocampus in our chronic rat TBI model, where the progenitor of this HBOT protocol was tested (Harch et al., 2007). HBOT-induced increased hippocampal blood vessel density in this model highly correlated with improved spatial learning and memory. In our subjects SPECT SPM analysis showed significant improvements in blood flow in the hippocampus, while our subjects achieved significant gains in memory. These blood flow and memory improvements seen in our subjects are consistent with a trophic effect of HBOT on chronic brain wounding in the hippocampus, and possible healing/reinnervation of denervated tissue (Bauman et al., 2009).
Other mechanisms may contribute to the HBOT effects seen in our study. A single hyperbaric oxygen re-oxygenation session causes prolonged excitability and neural plasticity of hippocampal neurons after exposure to hypoxia (Garcia et al., 2010), consistent with the Neubauer effect generated in this study. Repetitive HBOT has shown increased neurogenesis and cerebral blood flow in chronic global ischemia (Zhang et al., 2010). Zhang and associates administered repetitive HBOT 30 days after ischemic insult, similar to the 50-day delay in our animal model (Harch et al., 2007). Neurogenesis has been shown to occur in association with angiogenesis (Palmer et al., 2000). As mentioned above, angiogenesis is a known trophic mechanism of HBOT, and may be responsible for the increased blood vessel density seen in our animal model (Harch et al., 2007). HBOT has also been shown to cause the release of bone marrow stem cells into the peripheral circulation (Thom et al., 2006). Peripheral stem cells are known to cross the blood–brain barrier (Mezey et al., 2003).
The limitations of the present study were a lack of confirmation of post-injury brain MRI results in some subjects,
unblinded investigators (except for the SPECT brain imaging SPM analysis), and lack of a control group. The lack of confirmation of brain MRI findings in a few subjects could confound study results only by inadvertent inclusion of nonclinically-apparent neurological disease that was manifest on MRI alone. We believe this is a very remote possibility; these young men were highly fit pre-military, underwent regular fitness evaluations while in the military, and had no premorbid disqualifying conditions. All symptomatology commenced with the incident blast and was present continuously since the blast. Routine late MRI evaluations in mild to moderate TBI are usually negative, consistent with the majority of the scans in our subjects. We presume the few missing data points would similarly be normal or non-contributory.
Investigator bias and placebo effects possibly contributed to the magnitude of some of the effects we measured, but are unlikely to account for the majority of the effects or the consistency and magnitude of the effects seen across all domains, particularly SPECT. Investigator bias could be present in the P.I.’s symptom and physical exam recording, and in S.R.A.’s neuropsychological testing, but it does not explain the significant SPECT findings for which separate independent analyses, one of which was blinded, were performed by E.F.F. in North Dakota and D.A. and D.V.T. in California. None of the SPECT co-investigators interacted with the subjects, and they performed their analyses months after the subjects had completed their final imaging. Importantly, the blinded SPECT analyst, D.V.T., produced the most significant statistical results.
Placebo effects cannot be entirely ruled out; however, there are multiple arguments against this notion. Treatment effect size in two meta-analyses of randomized placebo-controlled trials versus observational studies performed on the same treatments has been shown to be very similar (Benson and Hartz, 2000; Concato et al., 2000). This suggests that placebo effects are overestimated in observational studies such as ours. Placebo effects on many of the cognitive measures in our study have been reported to be smaller than the changes we found with HBOT for FSIQ and WMS Visual Immediate and Delayed Memory (Doraiswamy et al., 2007), for Stroop Reaction Time (Calabrese et al., 2008), and for Stroop Color/Word raw score ( Jorge et al., 2010). The placebo effects reported on SPECT in psychiatric disease, in healthy individuals, and in neurological disease have shown focal changes in regional cerebral blood flow (Beauregard, 2009), most commonly in the inferior frontal gyrus, striatum, and rostral anterior cingulate cortex ( Jarcho et al., 2009). The global diffuse changes we measured have not been reported. In addition, it is highly improbable that a placebo effect could account for the multiplicity of differential changes on SPECT seen after 1 and 40 HBOTs using two different forms of mathematical/statistical analyses. Lastly, the parallel improvements in memory scores and hippocampal blood flow are inconsistent with a placebo effect.
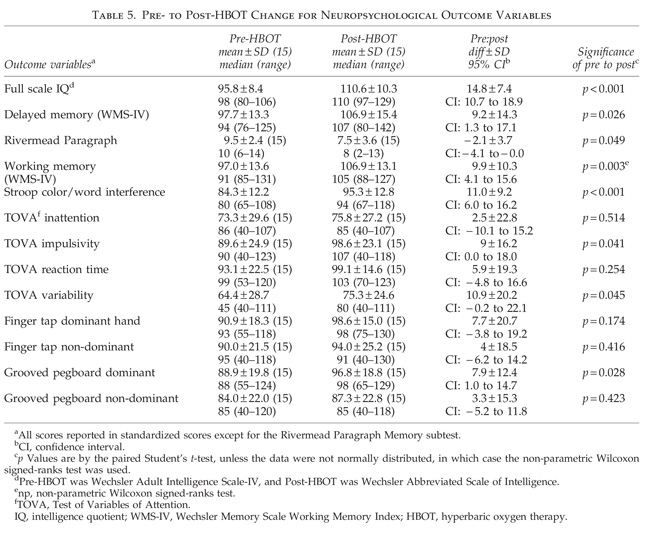
Test/retest practice effects could explain some of the cognitive improvements; however, practice effects do not fully explain our measured increases for seven reasons. (1) Practice effects on the WAIS-III FSIQ over a mean 34.6-day retest interval have been shown to be 2.0–3.2 points across all age groups, 6 points in the 16- to 29-year-old group, and decrease with age; our subjects averaged 30 years old (Tulsky and Zhu, 1997). They have also been shown to increase 6 points over 3-or 6-month retest times (Basso et al., 2002). Six points is 41% of the measured FSIQ increase on the WAIS-IV in our subjects. (2) The bulk of practice effects occur on the first retest (Bartels et al., 2010; Falleti et al., 2006), and our subjects had been cognitively tested at least once before our pre-HBOT testing session. Second and third retest (third and fourth tests) effects should have been smaller than 6 points. (3) Working memory has been shown to be among the most resistant to practice/retest effects (Bartels et al., 2010; Basso et al., 2002). Our subjects averaged a 9.9-point statistically significant improvement. (4) Practice effects are usually studied in normal individuals with intact memory function. Intact memory is a prerequisite for learning/practice effects. In individuals with impaired memory function, such as our subjects, practice effects may be less (Basso et al., 2002). (5) We used the alternate form WASI for the post-treatment IQ test in order to minimize practice effects. (6) A Stroop Color/Word score increase in a controlled HBOT study of chronic brain injury produced results similar to ours (Golden et al., 2006). (7) Stroop Color/Word test/retest effects across 1- and 2-week intervals are 3.83 points (Franzen et al., 1987), and our increase was 11.0 points.
Our results were achieved with half (40 HBOTs) of our normal protocol (80 HBOTs) on an accelerated twice/day schedule due to time and fiscal constraints. Through clinical experience, clinical research, and an animal pilot study that compared sham HBOT, 40, and 80 HBOTs (Harch et al., 1996b), we found greater cognitive and blood flow improvements (in an animal model; Harch et al., 2007), and clinical and blood flow improvements (in human cases) with 80 HBOTs, but the cases were primarily chronic moderate to severe TBI (vide supra). Neubauer and Golden (Golden et al., 2002) reported progressively greater blood flow in a case series of chronic severe brain-injured patients receiving 70 low pressure HBOTs. Recently, Wright and colleagues (2010) reported the effectiveness of our HBOT 1.5 ATA protocol in two airmenwith blast-induced PCS, using 40 and 80 HBOTs (for persistent symptoms after 40 HBOTs). Our subjects finished HBOT with partial improvement in their symptoms. It is likely that additional HBOT sessions would be beneficial.
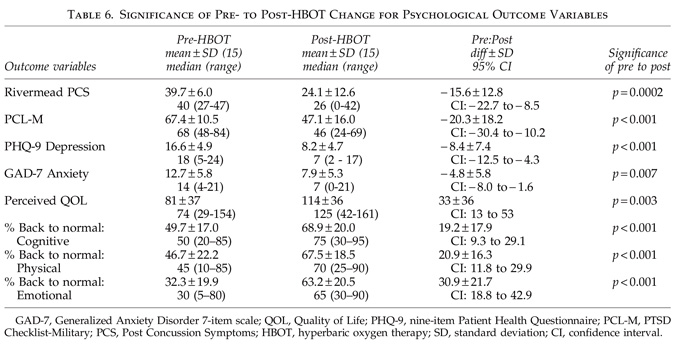
In conclusion, application of a lower-pressure protocol of 40 HBOTs at 1.5 ATA to a 16-subject cohort of military subjects with blast-induced chronic PCS and PTSD was found to be safe. One fourth of the subjects experienced transient clinical deterioration halfway through the protocol and one subject did not finish. Simultaneously, as a group the 15 subjects experienced notable improvements in symptoms, abnormal physical exam findings, cognitive testing, PCS and PTSD symptom questionnaires, quality-of-life questionnaires, depression and anxiety indices, and SPECT brain blood flow imaging that are inconsistent with the natural history of PCS 2.8 years post-injury. The symptomatic improvements were present at 6-month phone follow-up in 92% of subjects who reported improvement after 40 HBOTs. More objective psychometric testing and SPECT imaging were not performed to confirm the durability of the HBOT treatment effect. Sixty-four percent of the patients on psychoactive and narcotic prescription medications were able to decrease or eliminate use of these medications. These data are preliminary and need confirmation with larger numbers of subjects or with a stronger design such as a randomized or Bayesian study.
Acknowledgments
The authors thank The Marine Corps Law Enforcement Foundation, The Semper Fi Fund, The Coalition to Salute Americas Heroes, the Harch Hyperbaric Research Fund of the Baromedical Research Institute of New Orleans, Mr. Caleb Gates, New Orleans Natural Resource Group, Rubie and Bryan Bell, Martin and Margaret Hoffmann, John and Virginia Weinmann, Dr. Warren Thomas, Joan C. White, Health Freedom Foundation, Soldiers Angels, Operation Homefront Louisiana, The Audubon Society, Mr. Theodore Solomon, New Orleans Steamboat Company, the National WWII Museum, and Westwego Swamp Boat Tours for their generous donations. We thank Mr. Martin Hoffmann, ex-Secretary of the Army (President Gerald Ford) for his indefatigable fundraising efforts, Sean Bal and Ray Crowell, our hyperbaric technicians for their expert and safe delivery of hyperbaric oxygen therapy, Wanda Phillips for review of all of the study records, and Amy Trosclair of the BRI for overseeing the handling and disbursement of funds.
Author Disclosure Statement
Dr. Harch owns a small consulting company called Harch Hyperbarics, Inc., which has no contracts. For Dr. Andrews no competing financial interests exist. Juliette Lucarini, R.N. is a tenant in common ownership of Harch Hyperbarics, Inc. For Claire Aubrey, Dr. Fogarty, and Dr. Staab no competing financial interests exist. Dr. Pezzullo is an independent statistical consultant for whom no competing financial interests exist. For Dr. Amen and Derek Taylor no competing financial interests exist. Dr. Van Meter has a hyperbaric equipment leasing company and contracts with hospitals to provide hyperbaric medicine physician staffing.
References
- Andrykowski, M.A., Cordova, M.J., Studts, J.L., and Miller, T.W. (1998). Posttraumatic stress disorder after treatment for breast cancer: Prevalence of diagnosis and use of the PTSD Checklist-Civilian Version (PCL-C) as a screening instrument. J. Consult. Clin. Psychol. 66, 586–590.
- Ashburner, J., and Friston, K.J. (1999). Nonlinear spatial normalization using basis functions. Hum. Brain Mapp. 7, 254–266.
- Axelrod, B.N. (2002). Validity of the Wechsler abbreviated scale of intelligence and other very short forms of estimating intellectual functioning. Assessment 9, 17–23.
- Bartels, C., Wegrzyn, M., Ackermann, V., and Ehrenreich, H. (2010). Practice effects in healthy adults: a longitudinal study on frequent repetitive cognitive testing. BMC Neurosci. 16, 111–118.
- Basso, M.R., Carona, F.D., Lowery, N., and Axelrod, B.N. (2002). Practice effects on the WAIS-III across 3 and 6 month intervals. Clin. Neuropsychologist 16, 57–63.
- Bauman, R.A., Ling, G., Tong, L., Januszkiewicz, A., Agoston, D., Delanerolle, N., Kim, Y., Ritzel, D., Bell, R., Ecklund, J., Armonda, R., Bandak, F., and Parks, S. (2009). An introductory characterization of a combat-casualty-care relevant swine model of closed head injury resulting from exposure to explosive blast. J. Neurotruama 26, 841–860.
- Beauregard, M. (2009). Effect of mind on brain activity: evidence from neuroimaging studies of psychotherapy and placebo effect. Nord. J. Psychiatry 63, 5–16.
- Benson, K., and Hartz, A.J. (2000). A comparison of observational studies and randomized, controlled trials. N. Engl. J. Med. 342, 1878–1886.
- Bremner, J.D. (2007). Neuroimaging in posttraumatic stress disorder and other stress-related disorders. Neuroimaging Clin. N. Am. 17, 523–538.
- Calabrese, C., Gregory, W.L., Leo, M., Kraemer, D., Bone, K., and Oken, B. (2008). Effects of a standardized Bacopa monnieri extract on cognitive performance, anxiety, and depression in the elderly: a randomized, double-blind, placebo-controlled trial. J. Altern. Complement. Med. 14, 707–713.
- Carlson, C.F., Kehle, S.M., Meis, L.A., Greer, N., MacDonald, R., Rutks, I., Sayer, N.A., Dobscha, S.K., and Wilt, T.J. (2010). Prevalence, assessment, and treatment of mild traumatic brain injury and posttraumatic stress disorder: A systematic review of the evidence. J. Head Trauma Rehabil. Jul 13 [Epub ahead of print].
- Centers for Medicare and Medicaid Services. National Coverage Determination (NCD) Hyperbaric Oxygen Therapy (20.29). National Coverage Determinations (NCD) Manual, Publication Number 100-3, Chapter 1, Part 1, Section 20.29, effective date 6/19/2006. http://go.cms.gov/10J7QPV
- Clark, J.M. (1993). Oxygen toxicity, in: Chapter 6, The Physiology and Medicine of Diving, 4th ed. P. Bennett and D. Elliott (eds). W.B. Saunders: London, pps. 121–169.
- Clark, J. (2009). Side effects, in: Hyperbaric Oxygen Therapy Indications, 12th ed. The Hyperbaric Oxygen Therapy Committee Report. L.B. Gesell (ed). Undersea and Hyperbaric Medical Society: Durham, NC, pps. 217–218.
- Concato, J., Shah, N., and Horwitz, R.I. (2000). Randomized, controlled trials, observational studies, and the hierarchy of research designs. N. Engl. J. Med. 342, 1887–1892.
- Cyceron, Plateforme d’Imagerie Biomedicale. http://www.cyceron.fr
- Doraiswamy, P.M., Babyak, M.A., Hennig, T., Trivedi, R., White, W.D., Mathew, J.P., Newman, M.F., and Blumenthal, J.A. (2007). Donepezil for cognitive decline following coronary artery bypass surgery: a pilot randomized controlled trial. Psychopharmacol. Bull. 40, 54–62.
- Dougherty, G. (1996). Quantitative CT in the measurement of bone quantity and bone quality for assessing osteoporosis. Med. Eng. Phys. 18, 557–568.
- Falleti, M.G., Maruff, P., Collie, A., and Darby, D.G. (2006). Practice effects associated with the repeated assessment of cognitive function using the CogState Battery at 10-minute, one week and one month test-retest intervals. J. Clin. Exp Neurop. 28, 1095–1112.
- Franzen, M.D., Tishelman, A.C., Sharp, B.H., and Friedman, A.G. (1987). An investigation of the test-retest reliability of the Stroop Color-Word Test across two intervals. Arch. Clin. Neuropsych. 2, 265–272.
- Gaetz, M. (2004). The neurophysiology of brain injury. Clin. Neurophys. 115, 4–18.
- Garcia, A.J., Putnam, R.W., and Dean, J.B. (2010). Hyperbaric hyperoxia and normobaric reoxygenation increase excitability and activate oxygen-induced potentiation in CA1 hippocampal neurons. J. Appl. Physiol. 109, 804–819.
- Gavin, D.R., Ross, H.E., and Skinner, H.A. (1989). Diagnostic validity of the Drug Abuse Screening Test in the assessment of DSM-III drug disorders. Br. J. Addiction 84, 301–307.
- Gesell, L.B. (2009). Chair and Editor, Hyperbaric Oxygen Therapy Indications, 12th ed. The Hyperbaric Oxygen Therapy Committee Report. Durham, NC: Undersea and Hyperbaric Medical Society.
- Godman, C.A., Chheda, K.P., Hightower, L.E., Perdrizet, G., Shin, D.-G., and Giardina, C. (2009). Hyperbaric oxygen induces a cytoprotective and angiogenic response in human microvascular endothelial cells. Cell Stress and Chaperones December, DOI 10.1007/s12192-009-0159-0.
- Golden, Z., Golden, C.J., and Neubauer, R.A. (2006). Improving neuropsychological function after chronic brain injury with hyperbaric oxygen. Disabil. Rehabil. 28, 1379–1386.
- Golden, Z.L., Neubauer, R., Golden, C.J., Greene, L., Marsh, J., and Mleko, A. (2002). Improvement in cerebral metabolism in chronic brain injury after hyperbaric oxygen therapy. Int. J. Neurosci. 112, 119–131.
- Greenberg, L. (1996). Test of Variables of Attention Continuous Performance Test. Universal Attention Disorders, Inc.: Los Alamitos, CA.
- Hampson, N.B., Simonson, S.G., Kramer, C.C., and Piantadosi, C.A. (1996). Central nervous system oxygen toxicity during hyperbaric treatment of patients with carbon monoxide poisoning. Undersea Hyperb. Med. 23, 215–219.
- Harch, P.G., and Neubauer, R.A. (1999). Hyperbaric Oxygen Therapy in Global Cerebral Ischemia/Anoxia and Coma, in: Chapter 18, Textbook of Hyperbaric Medicine, 3rd revised ed. K.K. Jain (ed.). Hogrefe and Huber Publishers: Seattle, pps. 319–345.
- Harch, P.G., and Neubauer, R.A. (2004a). Hyperbaric oxygen therapy in global cerebral ischemia/anoxia and coma, in: Chapter 18, Textbook of Hyperbaric Medicine, 4th revised ed. K.K. Jain (ed). Hogrefe and Huber Publishers: Seattle, pps. 223–261.
- Harch, P.G., and Neubauer, R.A. (2009b). Hyperbaric oxygen therapy in global cerebral ischemia/anoxia and coma, in: Chapter 19, Textbook of Hyperbaric Medicine, 5th revised ed. K.K. Jain (ed). Hogrefe and Huber Publishers: Seattle, pps. 235–274.
- Harch, P.G., Fogarty, E.F., Staab, P.K., and Van Meter, K. (2009a). Low pressure hyperbaric oxygen therapy and SPECT brain imaging in the treatment of blast-induced chronic traumatic brain injury (post-concussion syndrome) and post traumatic stress disorder: a case report. Cases Journal 2, 6538. http://casesjournal.com/casesjournal/article/view/6538
- Harch, P.G., Kriedt, C.L., Weisand, M.P., Van Meter, K.W., and Sutherland, R.J. (1996b). Low pressure hyperbaric oxygen therapy induces cerebrovascular changes and improves complex learning/memory in a rat open head bonk chronic brain contusion model. Undersea Hyperb. Med. 23 (Suppl.), 48.
- Harch, P.G., Kriedt, C., Van Meter, K.W., and Sutherland, R.J. (2007). Hyperbaric oxygen therapy improves spatial learning and memory in a rat model of chronic traumatic brain injury. Brain Res. 1174, 120–129.
- Harch, P.G., Neubauer, R.A., Uszler, J.M., and James, P.B. (2009c). Appendix: Diagnostic Imaging and HBO Therapy, in: Chapter 44, Textbook of Hyperbaric Medicine, 5th revised ed. K.K. Jain (ed). Hogrefe and Huber Publishers: Seattle, pps. 505–519.
- Harch, P.G., Neubauer, R.A., Uszler, J.M., and James, P.B. (2004b). Appendix: Diagnostic Imaging and HBO Therapy, in: Chapter 41, Textbook of Hyperbaric Medicine, 4th revised ed. K.K. Jain (ed). Hogrefe and Huber Publishers: Seattle, pps. 471–485.
- Harch, P.G. (2002). The dosage of hyperbaric oxygen in chronic brain injury, in: The Proceedings of the 2nd International Symposium on Hyperbaric Oxygenation for Cerebral Palsy and the Brain-Injured Child. J.T. Joiner (ed). Best Publishing Co.: Flagstaff, pps. 31–56.
- Harch, P.G., Van Meter, K.W., Gottlieb, S.F., and Staab, P. (1994). HMPAO SPECT brain imaging and low pressure HBOT in the diagnosis and treatment of chronic traumatic, ischemic, hypoxic, and anoxic encephalopathies. Undersea Hyperb. Med. 21 (Suppl.), 30.
- Harch, P.G., Van Meter, K.W., Neubauer, R.A., and Gottlieb, S.F. (1996a). Use of HMPAO SPECT for assessment of response to HBO in ischemic/hypoxic encephalopathies, in: Appendix, Textbook of Hyperbaric Medicine, 2nd ed. K.K. Jain (ed). Hogrefe and Huber Publishers: Seattle, pps. 480–491.
- Hardy, P., Johnston, K.M., De Beaumont, L., Montgomery, D.L., Lecomte, J.M., Soucy, J.P., Bourbonnais, D., and Lassonde, M. (2007). Pilot case study of the therapeutic potential of hyperbaric oxygen therapy on chronic brain injury. J. Neurol. Sci. 253, 94–105.
- Hays, J.R., Reas, D.L., Shaw, J.B. (2002). Concurrent validity of the Wechsler abbreviated scale of intelligence and the Kaufman brief intelligence test among psychiatric inpatients. Psychol. Rep. 90(2), 355-9.
- Hoge, C.W., McGurk, D., Thomas, J.L., Cox, A.L., Engel, C.C., and Castro, C.A. (2008). Mild traumatic brain injury in U.S. soldiers returning from Iraq. N. Engl. J. Med. 358, 453–463.
- Hossman, K.-A. (1993). Disturbances of cerebral protein synthesis and ischemic cell death, in: Volume 96, Progress in Brain Research. K. Kogure, K.-A. Hossman, and B.K. Siesjo (eds). Elsevier Science Publishers: Amsterdam, pps. 161–177.
- Jarcho, J.M., Mayer, E.A., and London, E.D. (2009). Neuroimaging placebo effects: new tools generate new questions. Clin. Pharmacol. Ther. 86, 352–354.
- Jorge, R.E., Acion, L., Moser, D., Adams, H.P., and Robinson, R.G. (2010). Escitalopram and enhancement of cognitive recovery following stroke. Arch. Gen. Psychiatry 67, 187–196.
- Keane, T., Fairbank, J., Caddell, J., Zimering, R., Taylor, K., and Mora, C. (1989). Clinical evaluation of a measure to assess combat exposure. Psychological Assessment 1, 53–55.
- Kennedy, J.E., Jaffee, M.S., Leskin, G.A., Stokes, J.W., Leal, F.O., and Fitzpatrick, P.J. (2007). Posttraumatic stress disorder and posttraumatic stress disorder-like symptoms and mild traumatic brain injury. J. Rehabil. Res. Dev. 44, 895–920.
- King, N., Crawford, S., Wenden, F., Moss, N., and Wade, D. (1995). The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J. Neurology 242, 587–592.
- Kraus, M.F., Susmaras, T., Caughlin, B.P., Walker, C.J., Sweeney, J.A., and Little, D.M. (2007). White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain 130, 2508–2519.
- Kroenke, K., Spitzer, R.L., and Williams, J.B.L. (2001). The PHQ-9, validity of a brief depression severity measure. J. Gen. Intern Med. 16, 606–613.
- Lesak, M., Howieson, D., and Loring, D. (2004). Neuropsychological Assessment, 4th ed. Oxford University Press: New York, pps. 365–367, 776.
- Lidvall, H.F. (1975). Recovery after minor head injury. Lancet 1, 100.
- Lin, J.W., Tsai, J.T., Lee, L.M., Lin, C.M., Hung, C.C., Hung, K.S., Chen, W.Y., Wei, L., Ko, C.P., Su, Y.K., and Chiu, W.T. (2008). Effect of hyperbaric oxygen on patients with traumatic brain injury. Acta Neurochir. Suppl. 101, 145–149.
- Lipton, M.L., Gulko, E., Zimmerman, M.E., Friedman, B.W., Kim, M., Gellella, E., Gold, T., Shifteh, K., Ardekani, B.A., and Branch, C.A. (2009). Diffusion-tensor imaging implicates prefrontal axonal injury in executive function impairment following very mild traumatic brain injury. Radiology 252, 816–824.
- Marx, R.E., Ehler, W.J., Tayapongsak, P., and Pierce, L.W. (1990). Relationship of oxygen dose to angiogenesis induction in irradiated tissue. Am. J. Surg. 260, 519–524.
- MAST Revised. (2009). http://counsellingresource.com/quizzes/alcoholmast/index.html
- Mezey, E., Key, S., Vogelsang, G., Szalayova, I., Lange, G.D., and Crain, B. (2003). Transplanted bone marrow generates new neurons in human brains. PNAS 100, 1364–1369.
- Neubauer, R.A., Gottlieb, S.F., and Kagan, R.L. (1990). Enhancing ‘‘idling’’ neurons. Lancet, 335, 542.
- Neubauer, R.A., Gottlieb, S.F., and Pevsner, N.H. (1994). Hyperbaric oxygen for treatment of closed head injury. South. Med. J. 87, 933–936.
- Palmer, T.D., Willhoite, A.R., and Gage, F.H. (2000). Vascular niche for adult hippocampal neurogenesis. J. Comp. Neurol. 425, 479–494.
- Patrick, D.L., Danis, M., Southerland, L.I., and Hong, G. (1988). Quality of life following intensive care. J. Gen. Intern Med. 3, 218–223.
- Powell, J.M., Machamer, J.E., Temkin, N.R., and Dikmen S.S. (2001). Self-report of extent of recovery and barriers to recovery after traumatic brain injury: a longitudinal study. Arch. Phys. Med. Rehabil. 82, 1025–1030.
- PTSD Checklist-Military. (2009). http://www.nmcphc.med.navy.mil/deployment_health/frm_ptsdmilitary.aspx
- Rappaport, M., Hall, K.M., Hopkins, K., Belleza, T., and Cope, D.N. (1982). Disability rating scale for severe head trauma: coma to community. Arch. Phys. Med. Rehabil. 63, 118–123.
- Reitan, R.M., and Wolfson, D. (1993). The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. Neuropsychology Press: Tucson.
- Rossignol, D.A. (2007). Hyperbaric oxygen therapy might improve certain patho-physiological findings in autism. Med. Hypotheses 68, 1208–1227.
- Ryan, J.J., Carruthers, C.A., Miller, L.J., Souheaver, G.T., Gontkovsky, S.T., Zehr, M.D. (2003). Exploratory factor analysis of the Wechsler Abbreviated Scale of Intelligence (WASI) in adult standardization and clinical samples. Appl. Neuropsychol. 10(4), 252-6.
- Siddiqui, A., Davidson, J.D., and Mustoe, T.A. (1997). Ischemic tissue oxygen capacitance after hyperbaric oxygen therapy: a new physiologic concept. Plast. Reconstr. Surg. 99, 148–155.
- Spitzer, R.L., Kroenke, K., Williams, J.B.W., and Löwe, B. (2006). A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch. Intern Med. 166, 1092–1097.
- Sterr, A., Herron, K.A., Hayward, C., and Montaldi, D. (2006). Are mild head injuries as mild as we think? Neurobehavioral concomitants of chronic post-concussion syndrome. BMC Neurol. 6, doi:10.1186/1471-2377-6-7.
- Symonds, C. (1962). Concussion and its sequelae. Lancet 1, 1–5.
- Tanielian, T., and Jaycox, L.H., eds. (2008). Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. Center for Military Health Policy Research, The Rand Corporation: Arlington, VA.
- Thom, S.R., Bhopale, V.M., Velazquez, O.C., Goldstein, L.J., Thom, L.H., and Buerk, D.G. (2006). Stem cell mobilization by hyperbaric oxygen. Am. J. Physiol. Heart Circ. Physiol. 290, H1378–H1386.
- Tulsky, D., and Zhu, J. (1997). WAIS-III/WMS-III Technical Manual. Psychological Corporation: San Antonio, TX.
- Umile, E.M., Sandel, M.E., Alavi, A., Terry, C.M., and Plotkin, R.C. (2002). Dynamic imaging in mild traumatic brain injury: support for the theory of medial temporal vulnerability. Arch. Phys. Med. Rehabil. 83, 1506–1513.
- U.S. Navy Diving Manual, Revision 6, Vol. 1, Sec. 3-9.2.2.2. Symptoms of CNS oxygen toxicity, pps. 42–43. Chapter 3: Underwater Physiology and Diving Disorders. 3-9 Indirect Effects of Pressure on the Human Body. SS521-AG-PRO-010, 9010-LP-106-0957. Published by direction of Commander, Naval Sea Systems Command, 4/15/2008.
- Vorstrup, S. (1988). Tomographic cerebral blood flow measurements in patients with ischemic cerebrovascular disease and evaluation of the vasodilatory capacity by the acetazolamide test. Acta Neurol. Scand. 114 (Suppl.), 1–48.
- Wang, Z., Neylan, T.C., Mueller, S.G., Lenoci, M., Truran, D., Marmar, C.R., Weiner, M.W., and Schuff, N. (2010). Magnetic resonance imaging of hippocampal subfields in posttraumatic stress disorder. Arch. Gen. Psychiatry 67, 296–303.
- WAIS-IV. (2009). http://pearsonassess.com/HAIWEB/Cultures/en-us/ProductDetail.htm?Pid=015-8980-808&Mode=summary.
- WASI. (2009). http://pearsonassess.com/haiweb/cultures/enus/productdetail.htm?pid=015-8981-502
- Wechsler, D. (2001). Wechsler Test of Adult Reading (WTAR)-Manual. The Psychological Corporation: San Antonio, TX.
- Wilson, B., Cockburn, J., and Baddeley, A. (1985). The Rivermead Behavioural Memory Test. Thames Valley Test Company: Reading, England.
- WMS-IV. (2009). http://pearsonassess.com/HAIWEB/Cultures/en-us/Productdetail.htm?Pid=8895-800
- Woon, F.L., and Hedges, D.W. (2008). Hippocampal and amygdala volumes in children and adults with childhood maltreatment-related posttraumatic stress disorder: a metaanalysis. Hippocampus 18, 729–736.
- Wright, J.K., Zant, E., Groom, K., Schlegel, R.E., and Gilliland, K. (2009). Case report: Treatment of mild traumatic brain injury with hyperbaric oxygen. Undersea Hyperb. Med. 36, 391–399.
- Zhang, T., Yang, Q.-W., Wang, S.-N., Wang, J.-Z., Want, Q., Want, Y., and Luo, Y.-J. (2010). Hyperbaric oxygen therapy improves neurogenesis and brain blood supply in piriform cortex in rats with vascular dementia. Brain Inj. 24, 1350-1357.
Address correspondence to:
Paul G. Harch, M.D.
Hyperbaric Medicine Department
Department of Medicine
Section of Emergency and Hyperbaric Medicine
Louisiana State University Health Sciences Center
1542 Tulane Avenue, Room 452, Box T4M2
New Orleans, LA 70112
E-mail: [email protected]
Appendix
Standardized questionnaire:
- Energy level on 1–10 scale (10 was pre-LOC energy level, 0 is inability to get out of bed).
- Weight change since injury.
- Mood swings.
- Irritability/short temper.
- Mood, 1–10 scale (10 is happiest in life, 0 is not wanting to live).
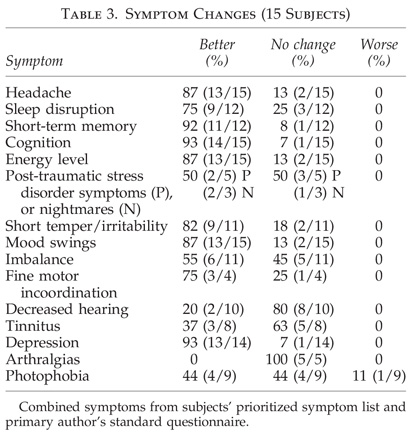
- Cranial and cranial nerve symptoms: headache, dizziness, visual symptoms, loss of hearing, tinnitus, vertigo, change in smell/taste, trouble talking, enunciating, swallowing, or chewing.
- Sensory symptoms: numbness, tingling.
- Motor: focal or generalized weakness.
- Incoordination: fine motor (hands/fingers), gross motor (tripping, stumbling, imbalance).
- Cognitive: trouble thinking/grasping ideas, organizing thoughts, decreased speed of thinking, confusion, problems following directions/instructions, difficulty expressing thoughts/word-finding, forgetfulness, misplacing/losing things, problems remembering old information or new information, losing one’s place in thought or conversation or while driving, going blank, staring episodes, feeling suddenly lost or disoriented, concentration/attention problems, difficulty writing, family or friends commenting on change in personality or behavior.
- Joint pain or swelling.
- Incontinence of bowel or bladder.
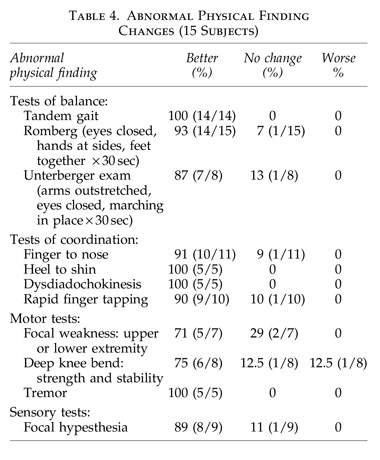
Neurological exam:
- Cranial nerves: II–XII.
- Deep tendon reflexes upper and lower extremities.
- Motor: tone, mass, tremor, deep knee bend, strength, tiptoe, and heel walking.
- Sensory: pinprick and touch in the four extremities.
- Gait: normal, tandem (slow and fast).
- Pathological reflexes: glabellar, snout, palmomental, grasp, suck, root, Hoffman, Babinski, clonus.
- Cerebellar: Romberg, finger tapping speed/rhythm, elbow flexion check response, finger-to-nose testing, heel-to-shin gliding, rapid alternating hand movementspalm/dorsal hand thigh slapping.
This article has been cited by:
- Sarah B. Rockswold, Gaylan L. Rockswold, David A. Zaun, Jiannong Liu. 2013. A prospective, randomized Phase II clinical trial to evaluate the effect of combined hyperbaric and normobaric hyperoxia on cerebral metabolism, intracranial pressure, oxygen toxicity, and clinical outcome in severe traumatic brain injury. Journal of Neurosurgery 118:6, 1317-1328. [CrossRef]
- George Wolf, David Cifu, Laura Baugh, William Carne, Leonardo Profenna. 2012. The Effect of Hyperbaric Oxygen on Symptoms after Mild Traumatic Brain Injury. Journal of Neurotrauma 29:17, 2606-2612. [Abstract] [Full Text HTML] [Full Text PDF][Full Text PDF with Links]
- Paul G. Harch, Susan R. Andrews, John C. Pezzullo. 2012. Response to the Letter to the Editor by Armistead-Jehle and Lee on Harch et al., “A Phase I Study of Low-Pressure Hyperbaric Oxygen Therapy for Blast-Induced Post-Concussion Syndrome and Post-Traumatic Stress Disorder”. Journal of Neurotrauma 29:15, 2516-2519. [Citation] [Full Text HTML] [Full Text PDF][Full Text PDF with Links]
- Patrick Armistead-Jehle, Dongwook Lee. 2012. Response to the Harch Group’s “A Phase I Study of Low-Pressure Hyperbaric Oxygen Therapy for Blast-Induced Post-Concussion Syndrome and Post-Traumatic Stress Disorder”. Journal of Neurotrauma 29:15, 2513-2515. [Citation] [Full Text HTML] [Full Text PDF] [Full Text PDF with Links]
- Hal S. Wortzel, David B. Arciniegas, C. Alan Anderson, Rodney D. Vanderploeg, Lisa A. Brenner. 2012. A Phase I Study of Low-Pressure Hyperbaric Oxygen Therapy for Blast-Induced Post-Concussion Syndrome and Post-Traumatic Stress Disorder: A Neuropsychiatric Perspective. Journal of Neurotrauma 29:14, 2421-2424. [Citation] [Full Text HTML] [Full Text PDF] [Full Text PDF with Links]
- Paul G. Harch, Susan R. Andrews, Edward Fogarty, Daniel Gregory Amen, Juliette Lucarini, Keith W. Van Meter. 2012. Response to Letter to the Editor by Wortzel and Colleagues. Journal of Neurotrauma 29:14, 2425-2430. [Citation] [Full Text HTML] [Full Text PDF] [Full Text PDF with Links]
- Donald J. Fogelberg, Jeanne M. Hoffman, Sureyya Dikmen, Nancy R. Temkin, Kathleen R. Bell. 2012. Association of Sleep and Co-Occurring Psychological Conditions at 1 Year After Traumatic Brain Injury. Archives of Physical Medicine and Rehabilitation 93:8, 1313-1318. [CrossRef]
- Semyon Slobounov, Michael Gay, Brian Johnson, Kai Zhang. 2012. Concussion in athletics: ongoing clinical and brain imaging research controversies. Brain Imaging and Behavior . [CrossRef]
- Daniel A Rossignol. 2012. Hyperbaric oxygen treatment for inflammatory bowel disease: a systematic review and analysis. Medical Gas Research 2:1, 6. [CrossRef]
- Daniel A Rossignol, James J Bradstreet, Kyle Van Dyke, Cindy Schneider, Stuart H Freedenfeld, Nancy O’Hara, Stephanie Cave, Julie A Buckley, Elizabeth A Mumper, Richard E Frye. 2012. Hyperbaric oxygen treatment in autism spectrum disorders. Medical Gas Research 2:1, 16. [CrossRef]
1Hyperbaric Medicine Department, Department of Medicine, Section of Emergency and Hyperbaric Medicine,
2Department of Medicine and Psychiatry, School of Medicine, Louisiana State University Health Sciences Center, New Orleans, Louisiana.
3Department of Radiology, University of North Dakota School of Medicine and Health Sciences, Bismarck, North Dakota.
4University of California, Irvine, School of Medicine, Amen Clinics, Inc., Newport Beach, California.
5Department of Medicine, Georgetown University Medical Center, Washington, D.C.
6Administrative Office, New Orleans, Louisiana.
Key words: hyperbaric oxygen therapy; post-concussion syndrome; post-traumatic stress disorder; single photon emission computed tomography; chronic traumatic brain injury; TBI; PCS; PTSD