Introduction
The concept of dosage of hyperbaric oxygen therapy (HBOT) derives from the definition of HBOT as a drug. Using the broad definition of HBOT by Harch and Neubauer (1), HBOT is the use of greater than ambient pressure oxygen as a drug to treat basic pathophysiologic processes/states and their diseases. Drug dosage of HBOT, therefore, is a function of baseline or reference ambient pressure, depth of pressurization, duration, frequency, air breaks, surface interval, number of treatments, idiosyncratic genetic patient factors, and time to intervention in the disease process which determines the pathological targets. All of these factors cause HBOT to be a narrow-window drug in chronic brain injury similar to digoxin and coumadin: too litfie maybe ineffective and too much can be toxic. In addition, oxygen is a respiratory metabolite: ;too little has serious metabolic consequences and too much can cause metabolic fatigue. Determining the proper dosage in a given patient with a specific or multiple diseases can be difficult. Ultimately, one wants the best dosage that improves the patient while doing the least harm.
HBOT has both acute and chronic effects (2). This paper will address only the chronic effects. Chronic effects of HBOT include fibroblast stimulation, collagen deposition, anglogenesis, epithelialization, and bone remodeling. This is most evident in shallow perfusion gradient wounds such as the classic homogenous wound of external beam radiation. In this animal and human model, Marx (3) has shown that intermittent exposure to HBOT induces the aforementioned chronic trophic effects to cause wound healing. The final level of tissue oxygenation after HBOT is approximately 80% of normal tissue, but the effect is durable for years. The unproven mechanism of the effect is thought to be secondary to transient elevation of tissue oxygen levels that results in a steep oxygen gradient that causes anglogenesis. Since 1995 this effect has been better characterized as signal induction where the drug HBOT, by elevation of tissue oxygen pressures, alone or in combination with other factors, signals the DNA to begin transcription of various gene sequences to mRNA (4,5,6). The mRNA is translated to proteins which cause trophic tissue changes, i.e., wound healing. These mechanisms are thought to be responsible for the HBOT-induced wound healing that is seen in a large variety of chronic non-healing wounds, such as arterial insufficiency, venous insufficiency, diabetic, radiation, sickle cell, vasculitic, and other ulcers.
The obvious question arises: why is this therapy not effective in the central nervous system.~ The answer is that this therapy is effective in central nervous system wounding, but is unappreciated. The major impediments to this appreciation and usage have been the attitude in medicine and neurologY that the brain, encased in the same body as all other human organs, is radically different from all of the other organs and hence, insensitive or resistant to rehabilitation, and that brain wounds respond to the same dose of HBOT as all other organs. Specifically, it has been assumed that the brain has a completely different set of tissue reactions to injury (the inflammatory reaction) than the rest of the body. This is false. While there may be subtle differences in acute injury between electrical, chemical, ischemic, hypoxic, traumatic, and other injuries, after a short period of time the inflammatory processes are very similar. More importantly, the chronic pathologY is nearly identical. Patients can have damage to glial cells, neurons, or blood vessels, but likely all three simultaneously. The brain cells can die or sustain lesser injury that allows them to live for prolonged periods in a less active state (7). These injured cells can exist in isolated tiny pockets of tissue or be distributed along shallow perfusion gradients, the ischemic penumbra (8), such as in Marx’s model. Exposure to HBOT can reawaken these neurons and allow them to function once again as normal neurons. HBOT may also stimulate repair of damaged neural tissue (9). Unfortunately, the exact dose of oxygen or HBOT necessary for this reawakening or repair is unknown. To this date oxygen remains one of the most difficult of all drugs to dose. It is the goal of this paper to explore the dosage of HBOT that seems to be appropriate for chronic brain injury rehabilitation. The argument constructed is derived from scientific literature, twelve years of HBOT treatment of over 40 different neurological conditions, 6 years of investigation/data generated under a community hospital based IRB-approved protocol, animal experimentation, personal experience, and the experience of other physicians, caregivers, patients, and family members of patients. This evidence is rooted in and blossomed from the dinical practice of medicine and so is a Bayesian process (10). Special attention will be directed to chronic pediatric brain injury. These data are a series of observations, not randomized prospective controlled data.
Methods
The author reviewed his twelve years’ experience treating chronic neurological conditions, approximately 450 patients, and all case reports/consultations phoned or e-mailed to him on patients nationally and internationally who developed untoward signs and symptoms during a course of HBOT for neurological conditions. Symptoms and signs were compared to known signs and symptoms of oxygen toxicity and reviewed to see if other common syndromes of undesirable HBOT side effects could be ascertained. The data is registered chronologically to show the Bayesian effect on the author’s practice and current protocol for HBOT in chronic brain injury. HBOT data were recorded according to the total dose of oxygen received by the patient on a given protocol. The total dose was calculated by multiplying depth atmospheres absolute x time (hours) x the number of HBOTs to give the number of atmosphere-hours (Ails). The cases were analyzed to see if certain doses were more or less likely to generate adverse signs and symptoms. Functional brain imaging was applied when available. Oxygen toxicity was defined as untoward neurological, cognitive, or constitutional signs and symptoms occurring in the setting of a course of HBOT. The signs and symptoms were of nervous system excitability (tremor, hyperreflexia, donus, etc.), those in the acronym VENTID (visual, ear, nausea, twitching, irritability, dizziness), and those listed in Table 6.1 of Chapter 6, OXYGEN TOXICITY, of The Physiology and Medicine of Diving, Fourth Edition, edited by Bennett and Elliott (11). Metabolic fatigue was defined as lethargy, lassitude, bradykinesia, or sleepiness occurring any time in the course of HBOT. Due to some overlap of symptoms of toxicity and other syndromes described below certainty of diagnosis was not possible in a few cases.
Table 6.1. Effects of central nervous system oxygen poisoning in normal men (adapted from Donald 1947, 1992)
Facial Pallor Unpleasant olfactory
Sweating sensations
Bradycardia Unpleasant gustatory
Choking sensation sensations
Sleepiness Respiratory changes
Depression panting
Euphoria grunting
Apprehension hiccoughs
Changes of behaviour inspiratory predomi-
fidgeting nance
disinterest diaphragmatic spasms
clumsiness Severe nausea
Visual symptoms Spasmadic vomiting
loss of acuity Vertigo
dazzle Fibrillation of lips
lateral movement Lip twitching
decrease of intensity Twitching of cheek and
constriction of visual field nose
Acoustic symptoms Palpitations
music Epigastric tensions
bell ringing Syncope
knocking Convulsions
Results
In May, 1990, a 46-year-old commercial diver (R.S.) presented with residual signs and symptoms of cerebral DCI four months after his last HBOT (31 treatments at 2.0, 2.4, and 1.5 ATA/90) and seven months after his diving accident (12). He was deposited in the author’s emergency room and hyperbaric unit by his brother and attorneys after the patient was intercepted on his planned homicide of McDermott diving company management personnel. The author unsuccessfully consulted various authorities for treatment and decided to treat according to a recommendation by Dr. Richard Neubauer at 1.5 ATA. Dr. Neubauer had also recommended 60-minute treatments, but the author settled on 90 minutes to be consistent with the standards of hyperbaric medicine wound healing protocols. The patient began a series of bid 1.5/90, 1.5/60, and qd 1.5/60 treatments over the course of the next two months. As he approached 80 treatments he experienced a regression in his improved neurological condition manifest by dizziness, restlessness, and increased agitation. These symptoms were complicated by his wife’s psychiatric problems and the death of a diving partner. His total dose of oxygen (depth x time x number of HBOTs) was 215 atmosphere-hours (AHs). He proceeded to an attempted suicide and was hospitalized along with his wife the next day in a psychiatric hospital for depression. While this was interpreted as a purely psychiatric decompensation at the time, in retrospect the partial regression of improvement, dizziness (particularly after chamber exit), restlessness, agitation, and finally, extreme confinement anxiety reaction were felt to be partly manifestations of oxygen toxicity. His total dose of oxygen was 215 AHs.
The following spring, 1991, a second diver (D.G.) was ¥eferred who was subsequently presented at the 1992 UHMS meeting in Bethesda, Md (13). The diver had cerebral DCI, and was treated five months after his accident and four months after three ineffective monoplace Table 6 HBOTs. The patient underwent 40 bid 1.5/90, 7d/wk HBOTs and then 40 bid 1.75/90, 7d/wk HBOTs and experienced dramatic improvement in his illness. His total dose of oxygen was 211 AHs. At the conclusion of treatment the patient was mildly irrational, hyperactive, and euphoric, and was diagnosed hypomanic by his psychiatrist. These symptoms resolved in 2-3 months and were later interpreted as oxygen toxicity (See above Table 6.1), resulting from a highly concentrated dose of 90 minute HBOTs.
Over the next three years multiple patients with chronic neurological conditions were treated on the 1.5/90 minute protocol, but mostly on a once/day, 6d/wk basis with good results. Many of these patients were entered on an IRB-approved protocol through JoElien Smith Hospital. One of these early patients was the first of the first five HBOT treated cerebral palsy children in North America (M.K.) (14). The patient was referred by Dr. Sheldon Gottlieb, University of South Alabama, and was the product of a precipitous induced post-term labor who was born with jitteriness, hypothermia, and a sub-arachnoid hemorrhage. At four years old he was hypotonic and unable to walk without torso stabilization. Following 80 1.5/90 bid, 5d/wk HBOTs in two blocks of 40 he was able to walk with fingertip support for balance and had improved hand fine motor function. Amazed at the child’s progress, the mother rented a home in New Orleans for the spring/summer of 1993 and requested prolonged additional treatment. (“More is Better?). The patient was treated another 113 times over 4 months and experienced partial regression of his fine motor gains, increased activity level, startle, and tremulousness, while showing some improvement in balance and cognition. His partial deterioration was interpreted as oxygen toxicity. His total dose of oxygen at symptom onset was poorly documented. Final dose of oxygen was 435 AHs. Three years later the patient received 15 1.5/60 HBOTs with cessation of drooling. After a two-week break he underwent another 19 treatments with a return of drooling. This regression of drooling likely was a manifestation of excess oxygen.
By Fall, 1993, a 19-year-old elective mute (14) with mild mental retardation and two hypoxic/ischemic brain injuries (J.K.) began two, 40 HBOT 1.5/60 bid, 5d/wk treatment courses under the author’s direction in the patient’s Texas hometown. The patient experienced significant neurological and behavioral improvement. After a 3-month break the patient continued with 40, 1.5 ATA qd HBOTs, but the dive time was increased to 90 minutes. Again, the patient improved neurocognitively and behaviorally. Three months later another round of 40 treatments at 1.5 ATA/90 caused improvement through 14 treatments then gross behavioral deterioration: hyperactive, rambunctious, and obnoxious. The patient seemed to calm by 28 to finish 40 treatments. Family noticed minimal overall improvement, but a burst of improvement in the weeks after HBOT ended. Total oxygen dose at 15 treatments
was 244 AHs. Three months later the patient undertook another 15 treatments at 1.5/90 qd. He was happy and relaxed by the 3rd treatment, became hyperactive and demanding on the 4th, and continued to deteriorate up to the 15th. Total oxygen dose by the 4th dive was 309 AHs. After receiving the mother’s report of the above the author recommended decreasing the treatment time to 60 minutes. Three months later the patient received 6 daily 1.5/60 HBOTs. Through five treatments the patient’s behavior improved then he became fidgety, hyperactive, and short-tempered by the 6th dive. Total oxygen dose was 343 AHs. Over the course of at least the next year, the patient received 4-5, 1.5/60 HBOTs every month with continued cognitive, motor, behavioral, and balance improvements. In summary this patient’s toxicity appeared to be the result of 90 minute dive times and a cumulative dose of HBOT that became manifest after a series of 6 HBOTs late in the course of extensive HBOT.
In April, 1994, 23 adults were poisoned with carbon monoxide. Six months later 4 of these patients (K.W.? 1LW., J.P., L.H.) embarked on a planned series of 40, 1.5/90 daily, 6d/wk. HBOTs for persistent neurological sequelae after completing an initial 40 delayed treatments. At nearly the identical point in the treatment course, 65-70 treatments, three of the four patients began to complain of dizziness, dysphoria, visual symptoms, and nausea during the last 30 minutes of treatments, or after exit from the chambers. These symptoms rapidly progressed to lethargy, fatigue, and increasing dysphoria. Treatment was stopped on all four patients. Total dose of oxygen was 157+ AHs. Consultation with Dr. Neubauer and other hyperbaric physicians revealed no experience with this constellation of symptoms. Given that the symptoms first appeared at depth during the last 30 minutes of treatment and immediately upon exit from the chamber it was assumed that these symptoms were oxygen toxicity and the 90 minute dive time was the cause of the toxicity. These four patients and a fifth CO patient were presented at the April, 1995, Gulf Coast Chapter UHMS Meeting in New Orleans (abstract available from the library of the author).
Simultaneously in Fall 1994, the author was consulted for treatment of a 32-year-old marine biologist (X.X.) who experienced cerebral DCI. The patient was treated with standard recompression and discharged to return to work. Due to a variety of cognitive complaints the patient was unable to return to work and 30 days post injury presented with work disability. The author recommended 1.5 ATA/60 minute treatments, but was reconsulted by the referring physician after three treatments left the patient with pallor, weakness, nausea, vomiting, and diaphoresis at the conclusion of each treatment. Further questioning revealed that the patient was diving with the other wound patients at 2.0 ATA using a blended mixture of hypoxic air to give 1.5 ATA oxygen at depth, but the treatments were two hours long. Total dose of oxygen was only 9 AHs. Shortening the treatments by locking the patient out at 60 minutes resolved the toxicity signs and symptoms. 27 treatments later the patient was substantially improved and returned to work.
In December 1994, a dramatic case of behavioral deterioration evolved in a 60-year old physician colleague of the author (R.I.). The patient had stroked two and one half years before and then, three months before HBOT, suffered a fall, LOC, tremor, and frontal lobe contusion. The patient received 40, 1.5/90 qd-bid HBOTs with noticeable clinical improvement. After a three-week break, he commenced a second round of 40 treatments. At 65 treatments the patient was relaxed and reporting improvement in his mentation, cognition, and motor abilities. By 75 treatments the patient experienced significant increase in activity/energy level (“energy to burn”), voracious appetite for reading, decreased sleep, and euphoria, followed by rapid increase in irritability, short-temper, anger, arguing, aggression, agitation, and irrational thinking/behavior in the next five HBOTs. Total dose of oxygen at this time was 169 AHs. Oxygen toxicity was felt to be the cause. Eight to ten weeks later the patient’s behavior and neurological condition returned to the peak level he achieved at 65 HBOTs.
In early 1995, the author proposed, designed, and wrote the protocol for the upcoming combined LSU/Galveston chronic traumatic brain injury/HBOT study (15). Immediately before submission he changed the protocol from 90 minute to 60 minute treatments to accommodate the most recent information on the CO patients, the 60-year-old physician and the 19-year old elective mute. At the conclusion of the 80 treatments in Galveston all five of the experimental patients were noted to be clinically improved by the patients themselves, their caregivers, families, and all of the treating physicians. As a result, forty additional treatments were demanded. The author cautioned against treating in large blocks after 80 HBOTs. Due to logistic demands the patients received another 40 consecutive 1.5/60 treatments. One patient experienced mild additional improvement, three were dysphoric, and the fifth grossly deteriorated. The patient who deteriorated was a 33-year-old male (S.B.) with traumatic brain injury who was experiencing bizarre uncontrollable behavior. Upon the patient’s mother’s insistence the patient was removed from the study at 110 treatments. When reviewing his SPECT imaging in Galveston the author noted the focal increase in blood flow at 80 treatments that became more exaggerated at 110 treatments. Follow-up imaging one year later (see Figure 1) demonstrated a deterioration in blood flow below the level of the patient’s baseline study in areas adjacent to the toxic increases in flow, implying an injurious effect of the oxygen toxicity. The behavioral deterioration took 8-10 weeks to resolve. Total oxygen dose at 110 treatments was 165 AHs.
Toxicity was similarly demonstrated in a group of near-&owning children. The first was an 9-year-old girl (B.McR.), five years after near-drowning (14). After evaluation and imaging by the author in August, 1993, the patient underwent 51 1.5/60 bid, 5d/wk HBOTs in California two months later with multiple motor and cognitive improvements. Three months later, she resumed treatment in Southern Illinois at 1.5/60 qd, 6d/wk for 29 HBOTs. Seven months afterwards she received treatment in her home state of Nevada at 2.0/120 qd. On the 19th treatment she seized at depth. Total oxygen dose was 196 AHs. Treatment resumed in New Orleans with the author 9 months later at 1.5/60 qd for 25 HBOTs. The patient again improved. She received additional treatment in Arizona 4 months later at 1.5/90 bid, 6d/wk. On the first HBOT she improved. With each successive HBOT she became increasingly fatigued and the mother reported extreme anxiety, hyperactive movement, and an appearance of discomfort in the chamber. She completed 24 HBOTs. By the 2nd HBOT, total oxygen dose was 238 AHs. Mom tried 14 more HBOTs at the same facility on the same schedule (?) six months later with no improvement. That was the patient’s final HBOT course. This case once again demonstrated effect of increasing depth (2.0 ATA), and time (120 and 90 minutes) late in a course of HBOT and its contribution to oxygen toxicity.
The second case was a 4-year-old boy (K.B.) 16 months post near-drowning who was evaluated in New Orleans June, 1993, then completed 80, 1.5/90 bid HBOTs in California from July to November (14). At 20-25 HBOTs the patient developed frequent myoclonus and was”pooping out;’ but showing increased cognitive, motor, and tone improvements. EEG after 80 treatments was negative. Total oxygen dose was 45 AHs. Subsequent EEG three months later while on break showed seizure activity, but myoclonus was decreasing. The child was placed on Tegretol with resolution of myoclonus. The mother returned to New Orleans five months after last HBOT and the treatment time was decreased to 60 minutes to minimize return of donus. He received 34 1.5/60 bid HBOTs. Myodonus decreased when HBOT began, the patient’s Tegretol was stopped at the 17th HBOT, and myoclonus returned at the 34th HBOT when the patient fatigued. Tegretol was resumed at this time. Neurological improvement plateaued at 30 HBOTs. Total oxygen dose was 231 AHs. After a three-week break he received another 24 HBOTs and again mild myoclonus and fatigue developed. Total oxygen dose was 267 AHs. Myoclonus abated on a three month break and mom discontinued Tegretol. HBOT was re-initiated at 1.5/60 qd for 35 treatments. Myoclonus returned with vigor at 32 treatments and a seizure occurred at 35 treatments which was associated with a”nightmare:’ Total oxygen dose was 312 AHs. The seizure/nightmares continued for another two months. After a four-month break mom obtained more HBOT at 1.5/60 qd for l0 treatments. While the patient improved and seizure frequency decreased, he became extremely irritable and began yelling at his mother frequently. Total oxygen dose was 327 AHs. Patient was lost to follow-up.
The third case was a 19-year-old young man (R.L.) who was two and one-half years post near-drowning. Seven weeks after his accident he obtained 30 2.0/90 minute bid HBOTs in Alabama with improved awareness and awakeness, but developed severe spasticity. Nineteen months later he began a series of 190 1.5-2.0/30-60 bid HBOTs over the next 10 months with progressive neurological improvement, but finally plateaued. At plateau he presented to the author for investigation of further dosing with HBOT. After imaging at 1.5/90 the patient resumed HBOT at 1.5/60 (?). Within 30 treatments the patient was independently ambulatory and had dear speech. By 275 HBOTs, the patient had”boundless energy:’ Mom placed a chamber in her home and went to work as a sales rep for a chamber manufacturing company. The patient continued to improve at phone followup of 350 HBOTs. Recommendation was made for careful observation based on the above experience with oxygen toxicity. This was
Figure 1: SPECT brain blood flow imaging of patient S. B. in LSU/UTMB Chronic Traumatic Brain Injury Study. Images are in statistical parametric mapping format and represent the images presented at three national meetings. The three columns on the left represent statistically significant increase in blood flow and the three columns on the right statistically significant decrease. The numbers on the far left refer to the number of HBOTs. “R’ is six months after the 80th HBOT. ~110” is at point of maximum oxygen toxicity and behavioral deterioration. ~IYR’ is one year following the 110th HBOT. Color map is purple, blue, green, yellow, orange, red, and white from the smallest to largest increase in blood flow on the left and smallest to largest decrease on the right. Note the large increase in flow in the red and yellow areas on the left hand images, Row 110, which largely disappear one year later. This is an improvement compared to the baseline images on Row 0 of the same side. On the right side, however, note the broad areas of decrease flow on Row 1YR which are much larger than the baseline areas of decrease flow on Row 0 of the same side. These areas of large decreases in flow are generally adjacent to the large increases in Row 110 on the left, implying a steal injury from toxicity on the left.
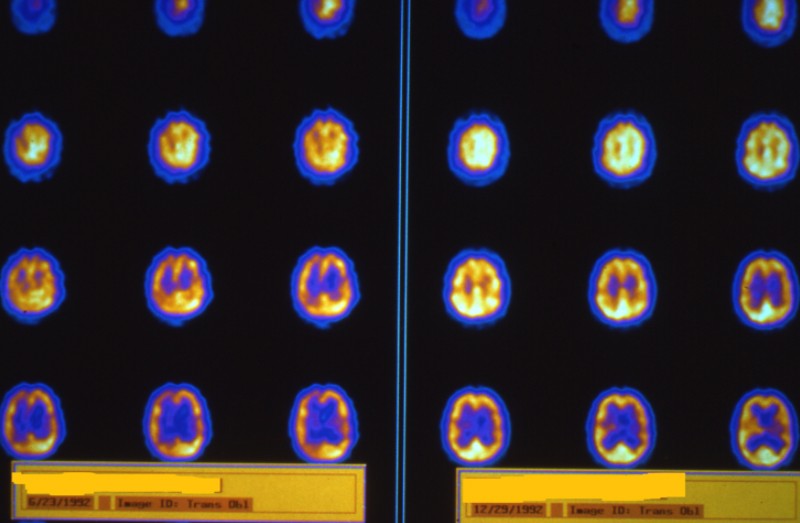
Figure 2: SPECT brain blood flow imaging performed immediately before HBOT in a 19-year-old severe traumatic brain injured female. Images are sagittal orientation and proceed from the right side of the brain in the upper left hand corner of the figure to the left side of the brain in the lower right hand corner. Images are in grayscale with highest blood flow registered as black and the lowest as white or faintest shades of gray. Note the marked decrease in flow to the top and posterior areas of the brain.
Figure 3: Repeat SPECT imaging on patient in Figure 2 soon after completion of 10 HBOTs at 2.0 ATA. Note the relatively large increase in flow to multiple areas of the brain. The radiologist’s interpretation was increase in flow consistent with “seizures”
Figure 4: SPECT brain blood flow imaging on a high resolution system at the author’s institution. Images are transverse orientation of a 7-year-old boy one year after chemical exposure and 11 months after new onset seizure disorder. The right side of the patient’s brain in each slice is on the viewer’s left. Images proceed from the top of the brain in the upper left hand corner of each scan date to the base of the brain in the lower right hand corner. Brain blood flow from highest to lowest is in white, yellow, orange, purple, blue, black. Baseline scan is on the right and repeat scan after 30 minute LPHBOT (1.5 ATA/60 minutes) is on the left, Notice the global smoothing of the pattern to one of homogenous brain blood flow after a single HBOT.
ignored. Follow-up phone conversation 1-2 years later revealed that the patient had experienced a gross behavioral deterioration with aggression, anger, irrational and uncontrollable behavior, necessitating institutionalization. The HBOT was continuing throughout this period to over 500 HBOTs, but was terminated after the conversation. Total oxygen dose was over 750 AHs. The patient has been lost to follow-up.
By 1998, low-pressure HBOT for CP was spreading to Canada and other areas in the United States. Multiple clinics opened offering this therapy, but most were using 1.75 ATA based on the 95% oxygen 1.75 ATA protocol in Sussex, England. Multiple parents began to send e-mails regarding their experience, a number of them reporting various problems. The first was a 4-year-old diplegic CP boy (M.N.) who received 22 ( 11 Rx’s/wk) 1.75 ATA (95% oxygen)/60 HBOTs in May, 1998, with good results. By September, he and his twin showed some mild regression and were taken for bid 1.75 ATA (100% oxygen)/60 (11 Rx/wk) HBOT in Canada. On the 56th HBOT he seized at depth; he had no prior seizure history. His total oxygen dose was 135 AHs.
The second child was a 12-year-old quad CP boy (E.M.) who experienced improvement with 40 1.75/60 bid 5d/wk HBOTs with two hour breaks between treatment at a free standing facility in the Eastern U.S. in June, 1998. He then received another 50 treatments in doses of 10, 20, 20 treatments at 1, 2, and 1 months intervals after the initial 40 treatments. Four weeks later he received another 33 treatments. Midway through this course he lost speech, attention, cognition, balance, coordination, became pale,increasingly lethargic, rubbery legged, started falling, and developed wheelchair dependence again. Mom described him as droopy and drunk. Total oxygen dose was 184 AHs. This resolved in 4-6 weeks and mom returned for another 10 bid treatments. The child experienced the same neurological deterioration which again resolved in 2-3 weeks. Total oxygen dose was 201 Ails. Two months after the last treatment the child received another 50 treatments. During this course his speech improved, but he became very fatigued and lethargic. Total oxygen dose was 289 AHs. Three weeks break did not resolve the fatigue, so mom returned for another 50 treatments (“More is Better”). During this time he became extremely energetic, talkative, happy, stopped taking naps, and was”wired” (the euphoric phase of toxicity). Total oxygen dose was 377 AHs. Two to three days after returning home he crashed with extreme lethargy, loss of speech, pallor, and global deterioration in neurocognitive function (withdrawal effect). The patient’s teachers were extremely concerned at the dramatic change in the child’s abilities. Three months later mom demanded a decrease in pressure to 1.5 ATA and the child received 30 treatments. This was well tolerated and the child improved speech, balance, motor, and cognitive functions. Two months later the child received another 18 treatments, had further improvements, but on the last day developed severe muscle spasms in his good leg. On examination in the ER he was found to have donus in a number of extremities. Total oxygen dose was 449 AHs. Metabolic workup was negative. In retrospect mom noticed that during some of his treatment courses he would develop anger and aggression along with the other symptoms noted above. The mother also noticed that multiple other children at this treatment facility were manifesting symptoms and signs similar to her son’s. The case illustrates multiple episodes of toxicity and withdrawal.
In 1999, more cases were catalogued who developed problems with low-pressure HBOT. The first was a two-year-old shaken baby boy (M.B.) who in April received 38 bid 1.75/60 HBOTs 22 months after his injury. His seizure frequency and medication requirement decreased noticeably. Two months later the child underwent another 40 (?) 1.75/60 HBOTs. During this second round of treatment the child experienced an equally dramatic rebound in seizure frequency which further worsened to a new plateau much worse than his original baseline upon his return home. His dose of oxygen was 137 AHs.
Also in April, 1999, an 18-month-old quad CP (S.T.) gift began 40, 1.5160 bid HBOTs, took a one month break, then finished 37 more treatments. Over the next six months, she received 13, 11, 12, and 13 treatments every 6-8 weeks. On the next round of treatments she became anorexic, “wild” with extreme exacerbation of tone and extensor posturing after the sixth treatment. This abated upon cessation of HBOT. Her total dose of oxygen was 198 AHs. Six weeks later she received another 7 treatments without incident, followed by a single treatment 8 weeks later on 9/25/00. After this treatment, mom described her as”wired;’ high tone, four hours sleep, hyperactive. Total dose was 210 AHs. Simultaneously and in the subsequent 3 months, her seizure frequency changed in character and frequency from one severe grand mai every 1-3 months requiring ER treatment to one mild seizure/day. By January 30, 2001, seizures were 4-6/wk and mild. The patient underwent six qd 1.25/60 HBOTs. After the fifth HBOT, she experienced some twitching which recurred in the chamber on the sixth HBOT. On return in six weeks the patient was seizure free, but had donus on exam. Over the next two months she had variable myodonus and occasional “lesser” seizures about once/2 wks. She then received four qd 1.25/60 HBOTs without incident. It appeared that the reduction in pressure mitigated the seizures which were a manifestation of oxygen toxicity.
In May, 1999, a 2-year-old boy with hypoxic ischemic encephalopathy (J.C.) began 1.25160 bid 6d/wk (once on Saturdays) HBOTs. The pressure was increased in increments to 1.5 and finally 1.75 ATA as the child’s seizure frequency decreased from 400-500/day to 100+. Simultaneously, the seizures became more prolonged and severe. By the 94th total treatment, now at 1.75 ATA, the child was admitted to the hospital to begin the ketogenic diet because of the severity of his seizures. Total dose of oxygen was 141 AHs. One week later he returned to HBOT at 1.25160 qd, 6d/wk for 35 more treatments. Seizures progressively decreased to 70/day and the child continued to improve neurologically. One year later the child began HBOT at 1.25/60 qd and bid for 30 treatments followed by a maintenance schedule 3-Sx/wk intermittently. Seizures now only occur on awakening or falling asleep or when the child is extremely fatigued. This case illustrated the detrimental effect of the increase in pressure to 1.75 ATA and the beneficial effect of the reduction to 1.25 ATA.
Another exacerbation in seizures was reported by a mother of a young boy (X.Y.) who underwent HBOT at a clinic on the East Coast of the U.S. in 1999. The child began 1.75/60 bid 5-6d/wk HBOT and sustained a marked increase in seizure frequency. Despite the worsening of seizures HBOT continued to approximately 40 treatments. As the mother continued to complain and seek information on the facility’s internet chat room she was excluded from the chat room by the owner of the facility and subsequently called the author for a consultation on her son’s dilemma. Total oxygen dose was 8 AHs at time of deterioration of seizures
A similar change in seizures occurred in July, 1999, in a 7-year-old boy (S. M.) with microcephaly, developmental delay, and seizures. He underwent 1.5/60 qd-bid HBOTs. Seizures decreased from 5/day to 0-1/day, increased dramatically at 28 HBOTs, then decreased to 0-1/dayby 40 with the development of clonus. Upon resumption of HBOT after a three-week break, seizures increased in the first 7 HBOTs and changed to a more severe type. This abated until 73 HBOTs, where another spike in seizures occurred which similarly abated. Two days after the 80th HBOT seizures increased to 10 severe/day. Total dose of oxygen was 120 AHs. This improved over the ensuing three months and further decreased upon reinstitution of HBOT. However, one day after the 10th treatment the patient experienced numerous severe seizures. Two weeks later the seizures became more intense. Five weeks later the patient was having 10 severe seizures/day. The patient’s neurologist added a new anticonvulsant and began to wean the other four. Seizures improved noticeably. Two months later in August, 2000, the seizures worsened again and were found to be secondary to low grade fevers secondary to reflux with aspiration during sleep. The head of the patient’s bed was elevated with resolution of the fevers and regression of seizures. The exact etiology and recent deterioration in seizures is unclear, but is possibly a combination of hyperbaric oxygen therapy and febrile episodes secondary to recurrent aspiration.
An additional two patients with chronic oxygen toxicity were the author’s patients. The first was a 3-year-old gift with athetoid CP (J. S.) who in October, 1999, received 80 bid 1.5/60 HBOTs in two blocks of 40, then 12, 10, 10, 10, and 10 bid over the next eight months from May, 2000, to January, 2001. On 2/26/2001 she began three bid 1.5/60 HBOTs and became extremely jittery after the third dive. This lasted one day. HBOT was discontinued. Total oxygen dose was 203 AHs. She subsequenfiy resumed HBOT at 1.25 ATA for a short period without incident. The second child is a 10-year-old girl with quad CP (W.B.) who underwent 40, bid 1.5/60 HBOTs followed two months later by 25 on the same schedule. She developed lethargy, startled to loud noises, and began biting multiple individuals. Examination revealed diffuse hyperrefiexia and clonus. Total oxygen dose was 97 AHs.
In January, 2001 a patient with dystonia was brought to the author’s attention. The patient is a young girl (I.A.) who underwent forty 1.75/60 bid HBOTs and experienced a marked improvement in dystonia and mental alertness. One month break ensued in which the patient completely regressed. An additional 20 1.75/60 bid HBOTs were administered and again the patient improved’ but regressed upon cessation. After approximately a one month break the mother was more determined to ensure durability of the hyperbaric-induced improvements and obtained 90 1.75/60 bid, 7 days/wk HBOTs. During this time the patient became “very energetic, active, healthy, and relaxed with improvement in dystonia again:’ A three week break followed and during the second week the patient had a dramatic reversal where she became extremely rigid, twisted, and “her legs are turning, her pelvis is deviated, her left hip.., she is not eating well:’ Mother then consulted a hyperbaric Internet chat group and became aware of author’s recommendations for lower pressure HBOT. The patient received a lower pressure dose of HBOT and experienced partial relief, but still had high tone. The hyperbarics was followed with six weeks of physical therapy and the patient had slight additional improvement. She then obtained additional HBOT at 1.27 ATA/60 qd, 5 days/wk for 7 treatments combined with physical therapy. The patient experienced marked improvement with reduction in tone, dystonia, and the resolution of the negative effects described during the withdrawal period above. The occurence of increased energy and activity was interpreted as oxygen toxicity. Total oxygen dose was 262 AHs.
By 2000/2001 the author had accumulated a series of patients who had experienced adverse symptoms soon after initiating HBOT. The first was the marine biologist from Texas (X.X.) treated in 1994/95 described above. While treated at 1.5 ATA, the precipitating HBOT feature of her toxicity was the 120-minute chamber runs. The second patient is the above child with seizures, patient x.y. The third patient was a 58-year old man (W.C.) three years after aneurysm rupture who began HBOT at 1.75/60 bid, 7d/wk. After three treatments the pressure was raised to 2.0 ATA for five HBOTs, 2.5 ATA for 11, 2.8 ATA for 1, then 2.5 for the remaining 5 treatments for a total of 25 HBOTs. While experiencing neurological improvement he became wild on the 20th treatment within minutes at depth. Air switch resolved the symptoms, but they resumed on oxygen breathing and the patient became incoherent. Total oxygen dose was 46 AHs. He had no further improvement on the remaining five treatments. Once home he lost all of his neurological gains in 2-3 weeks and his behavior deteriorated progressively over 2-3 months. Five to six months later he resumed a course of 40, 1.75/60 bid 7d/wk HBOTs. The patient was noticeably improved between the 10th and 15th treatments, but he was developing increasing oxygen intolerance at depth. By the 35th he was aggressive, angry, fatigued, and lethargic necessitating discontinuance of the HBOT course. Total dose of oxygen was 109. Once home he immediately went “berserk;’ according to the wife, with aggression, confusion, anger, and complete regression of neurological gains. Patient underwent extensive neurological workup which was negative. Three months later in 3/01 the wife consulted the author. It was felt that the patient had become oxygen toxic and was experiencing a type of withdrawal from HBOT and similar to the young girl immediately above (I.A.). The patient was prescribed 10 HBOTs at 1.5/60 which resulted in improved behavior. 10-15 days post HBOT the patient began to improve neurologically. He continued to advance over the ensuing 3 months, but did not return to his peak during the first course of HBOT. By July, 2001, three and one half months after last HBOT the patient became incoherent and incontinent. Hospital admission with extensive work-up, including unchanged CT of the brain was negative. When the patient’s wife administered oxygen by nasal cannula at 1L/min and then 4-5L/min the patient improved to his baseline and was discharged home.
The fourth patient is a 2-year-old boy (K.M.), 5 months post near-drowning who started HBOT this year at 1.75/60. The second treatment was at 2.4 ATA, the next 17 at 1.75, and final of the first 20 was at 2.0. The mother reported that on the 2.4 and 2.0 ATA dives “Kurtie cried a lot as though he was in a lot of pain and was very stressed:’ Since initiation of HBOT her son has developed”terrible shaking in his legs and chin and arms …. like he had do-nis.., which he never did before:’ Total dose of oxygen was 4.1 AHs at symptom onset. Despite this the child improved neurologically. The donus abated when HBOT ended.
The fifth patient is a 44-year-old man (B.A.) with anoxic brain injurywho began bid HBOT in 2/01 at 1.5/45. The second treatment was at 1.75/60, the third at 1.75/90, and the next four at 1.75/60. After the second HBOT the patient was incoherent, lethargic, and couldn’t eat. He worsened over the next 5 treatments until he was unable to walk, began to drool, and lost all of his neurological gains of the past two years. Total dose of oxygen was 2.9 AHs at symptom onset. The author was consulted about this patient and 1.5/45 HBOTs were recommended. One of these was performed followed by two HBOTs at 1.5/60. The patient’s mother and physician stopped treatment. The patient remained in his deteriorated condition with,little improvement at last phone followup five months after HBOT.
The sixth patient is a 19-year-old young woman (E.B.) who was involved in a severe MVA. Five months post injury she underwent 10, qd 2.0/90 HBOTs alongside woundcare patients in a multiplace chamber. Despite halo removal, C5-6 fusion, G-tube revision, Baclofen pump insertion, and for medication changes she experienced some calming in the chamber and a few hours post each HBOT. HBOT was terminated because of”no significant improvement:’ SPECT was performed before and after the course of each HBOT. The second SPECT was interpreted as “seizures.” Total dose of oxygen was 30 AHs. Patient is now receiving HBOT at the author’s facility at 1.5/60 bid. On July 19, 2001, she completed her first round of 40 treatments without complications. She experienced improvement in a number of functions. SPECT before this course of HBOT showed resolution of the toxicity.
The seventh patient is a 15-year-old boy (B.M.) with an idiopathic seizure disorder who began HBOT bid, 6d/wk at 1.7/60 for 23 treatments. On the first HBOT, the child hyperventilated, seized, became more lethargic, hyper-ventilated, seized again, and vomited. The treatment was immediately terminated. In the ensuing treatments seizures reproducibly occurred 20 minutes into the treatment and became more severe and more frequent. Reducing the pressure to 1.5 ATA on the 19th-23rd HBOTs was ineffective. Total oxygen dose was 1.7 AHs at symptom onset. Two weeks after the last HBOT the patient went into status epilepticus and two weeks hence underwent hemispherectomy. He has had eight subsequent seizures from February to July 13, 2001.
While at the conference presenting this paper, “the author received a phone call from the mother of the eighth patient;’ a 21-year-old male who had experienced a severe traumatic brain injury with diffuse axonal injury, sub-arachnoid hemorrhage, epidural hematoma, and Le Fort III fracture in February 1993. His hospital course was complicated by massive cerebral edema and elevation of intracranial pressures resulting in prolonged hospital stay. A year after injury the patient developed petit mai seizures which progressed to grand mai seizures a year later. Shortly thereafter he was discovered to have a frontal lobe abscess and nearly expired. He underwent three surgeries including a drainage procedure, repair of ethmoid sinus fracture, and then repeat drainage procedure for recurrence of the abscess. In November, 2000 the patient proceeded to Canada for hyperbaric oxygen therapy. He underwent eight 1.5/60 bid, 6 days/wk HBOTs without improvement. The pressure was increased to 1.75 ATA/60 bid, 6 days/wk and by ten treatments the patient had lost his balance and ability to walk. He broke furniture and a toilet in the hotel room and became irritable and more impulsive. Irritability increased by thirty treatments as did the patient’s anger, aggression, and belligerence. He was most agitated in the chamber at depth. At 39 treatments the mother brought the patient home unable to walk, with the above-noted problems. In the ensuing weeks
his agitation increased over a two month period, his coordination deteriorated globally, especially fine motor coordination, as evidenced by inability to feed himself. In the ensuing nine months the patient had slight improvement in balance and agitation but was still unable to walk, impulsive, inappropriate, and unable to feed himself properly. The patient presented to New Orleans and underwent a sequence of SPECT brain imaging, single hyperbaric treatment at 1.25 ATM60, and repeat SPECT brain imaging. The second SPECT scan showed a greater than 250% increase in brain blood flow and a noticeable smoothing of the abnormal pattern. The patient underwent a course of 1.25/60 bid, 5-day per week HBOTs with progressive improvement in behavior and cognition and a decrease in impulsivity and inappropriateness. Coordination and balance were slightly improved. Total dose of oxygen at onset of acute toxicity was 29 AHs. Total dose of oxygen at onset of withdrawal was 67 AHs.
In summarizing the above patients three separate symptom complexes were apparent: acute oxygen toxicity which occurs early in the HBOT course, chronic oxygen toxicity which occurs after a series of HBOTs, and a withdrawal syndrome occurring after abrupt cessation of a prolonged course of HBOT. In addition, a fourth syndrome of simple metabolic fatigue was obvious, but not recorded in this paper. The cases in the three groups are presented in the following tables:
Discussion
As defined above, HBOT is a drug. Increasing doses of HBOT can be toxic and oxygen toxicity is well known at 3.0 ATA and higher. Oxygen can be toxic at lower doses~also, but requires longer durations of exposure (11). A review of a series of the author’s patients and consultations randomly and sequentially accumulated over the past 12 years shows that signs and symptoms consistent with classical descriptions of oxygen toxicity do in fact occur at oxygen pressures under 2.0 ATA. The 37 examples (32 cases) were sorted into two separate categories of toxicity including acute oxygen toxicity, chronic oxygen toxicity, and a third syndrome of drug withdrawal from HBOT.
The cases of acute oxygen toxicity in Table 1 occurred in both adults and children with a variety of diagnoses at pressures of mostly 1.7 ATA and higher. The lone exception was the diver at 1.5 ATA, but the treatments were 120 minutes long. The reduction in treatment time to 60 minutes in this diver resolved the toxicity and allowed the patient to rehabilitate with HBOT. This case suggests that treatment times at this length at 1.5 ATA in chronic neurological injury are detrimental. The rapid onset of the negative effects in all of these chronic neurological cases at such low pressures for such short durations (60 minutes), however, implies a type of detrimental effect different from the true oxygen toxicity that is seen at much higher pressures. Vasoconstrictive shifts in brain blood flow may account for this phenomenon. Hyperoxic vasoconstriction could effectively steal blood flow from damaged areas rendering them more ischemic and dysfunctional. The deterioration in at least three of the cases was not immediately reversible and suggests a lasting negative effect: the 44 y.o. M who took months to partially recover two years’ of neurological gains, the 15 y.o. M whose seizures progressed to status epilepticus and hemispherec-tory, and the 21 y.o. M who lost gait, balance, and developed extreme agitation and belligerence, much of which persisted at least nine months until application of lower hyperbaric oxygen therapy.
The second syndrome was chronic oxygen toxicity. The cases were divided into those resulting from 1.5 ATA HBOTs and those from 1.75 ATA HBOTs, Tables 2 and 3, respectively. In the 1.5 ATA group ( 14 patients) the patients included both adults and children with a variety of diagnoses. Toxicity was clustered at 65-80 HBOTs (6 patients), 110-135 HBOTs (6 patients), and 2 patients at 194 and 305. No conclusions could be drawn on 60 vs. 90 minute treatments, qd vs. bid, total oxygen dose, or any other factors. The toxicity occurred at an average of 119 HBOTs. In contrast, the 1.75 group (8 patients) was mostly younger (probably an artifact of current off-label practice), but also with a variety of diagnoses. The patients required an average lesser number of HBOTs to generate toxicity (91), but they occurred more frequently after long strings of treatment. Three of these toxic episodes followed escalation of treatment pressure from 1.5 ATA to 1.75 (2 patients) or 2.0 ATA ( 1 patient). Lastly, four of the eight manifestations of toxicity involved first time seizures (2 patients) or worsening of seizures (2 patients). In a number of cases the toxicity took 8-10 weeks to resolve. In the only individual with follow-up SPECT brain imaging a deterioration in blood flow adjacent to the toxic areas was apparent one year after the event. Clinical correlation was not available.
The third syndrome, withdrawal, was characterized by a gross deterioration in global neurocognitive function and behavior within days of cessation of HBOT. All four episodes occurred in patients diving at 1.75 ATA, and two of the four after long strings of treatment. This pattern implies an habituation drug effect from which the patient withdraws. Possibly the detrimental signs and symptoms result from shifting blood flow in the acute withdrawal period. Interestingly, in the two cases in which the author intervened to direct treatment, the syndrome was ameliorated by reinstitution of HBOT at a lower pressure. In the fourth case, C.B., similar lower pressure HBOT after a nine month hiatus relieved some of the patient’s symptomatology. This mimics the pattern of acute withdrawal from other known habituating drugs such as narcotics, barbiturates, alcohol, etc. Such an effect has been described as an acute hyperbaric oxygen withdrawal by Donald (16) and termed the off-effect. The above syndrome would appear to be different from the off-effect in that this syndrome is more long-lasting, possibly permanent, and only seems to occur after prolonged HBOT.
Acute oxygen toxicity is felt to be benign, imparting no long term damage to animals and humans (17). It is characterized by dramatic increases in cerebral blood flow due to a breakdown in protective hyperoxic vasoconstriction (18,19). Generalized convulsions usually follow. Increased blood flow was seen on SPECT brain blood flow imaging in the 19-year old severe traumatic brain injured (TBI) young woman after 10 2.0/90 minute HBOTs. This had resolved nearly two years later. It was also captured on SPECT brain imaging in the 33-year- old TBI patient before his subsequent florid behavioral deterioration and concomitant worsened increase in SPECT blood flow (Figure 1 above). In this patient, however, repeat SPEGT brainone year later showed resolution of the toxic areas to a new baseline which was improved over his pre-HBOT SPECT levels, implying a benefit from HBOT in these areas. Simultaneously, adjacent areas showed deterioration one year later compared to the pre-HBOT levels, implying injury. This pattern has been described with 8 daily exposures to 5 ATA oxygen (20). The areas of lower blood flow have been shown to develop necrosis (21). These studies imply a permanent injury to the brain from oxygen toxicity. One possible explanation is a steal mechanism.
Limitations of the above data are that they are both random and sequential. The true numerator and denominator, the number of toxic events and total low-pressure HBOT chronic neurological cases, respectively, is unknown. The data was accumulated and assimilated in a Bayesian fashion, allowing the author to incorporate past and evolving events into future changes in protocol. Specifically, when treatment time was decreased from 90 to 60 minutes, oxygen toxicity was markedly less frequent in less than 120 treatments. As treatment exceeded 80 HBOTs, patients seemed to saturate with lesser and lesser numbers of treatments or required a reduction in pressure to 1.25 ATA to mitigate toxicity. Seizure disorders seemed to develop toxicity with earlier numbers of treatments and responded with a similar reduction in pressure. It may be prudent to start and stay at 1.25 ATA in these patients, especially if one contemplates treating in prolonged fashion. Unfortunately, the ideal dose for any patient is unknown.
Part of the answer to finding the correct dose for a given patient may lie in the answer to the questions, “What are the pathological targets of treatment and what is the mechanism of treatment?” All of the data in hyperbaric medicine for chronic wounding strongly suggests that the drug hyperbaric oxygen acts as a signal inducer of DNA. While no one has investigated the minimum amount of time under a single HBOT to cause signalling, the above data suggests that 90 minutes is too long and 30 minutes may be all that is necessary (see R. L. above). In addition, two of the author’s recent cases with SPECT brain imaging before and after a single HBOT also suggest that 30 minutes is sufficient to cause acute changes in brain blood flow and metabolism after a single low pressure HBOT in chronic brain injury. (One of these cases is presented in Figure 4) The mechanisms are very complex and likely multiple, as suggested by the recent Harch study (9) where repetitive low-pressure HBOT was found to reduce blood volume requirements in normal brain and repair injured neural tissue in a chronic TBI animal model.
In the author’s experience the ideal protocol is indeterminate because of the idosyncratic nature of each patient’s brain injury and his/her response to HBOT. Ultimately, the best approach to dosing the drug HBOT is to practice good medicine and observe the patient carefully. After 12 years of attempting to observe each patient carefully the best recommendation is to start HBOT for chronic brain injury at 1.5 ATA/60 minutes once/d, 6d/wk for 40 treatments, unless the patient has an active seizure disorder. For seizures start at 1.25 ATA. After 40 treatments, take at least a one month break, then deliver another 40 treatments. At the conclusion of 80 treatments, carefully reassess the patient and proceed with lesser numbers of HBOTs in shorter courses at varying intervals determined by the patient’s response to treatment and any untoward side effects. Should toxicity develop take a longer break and drop the pressure and/or time, once treatment resumes. Follow key signs and symptoms. Add additional therapies once the patient has shown an unequivocal response to HBOT, and use SPECT brain imaging from the outset as an indexing tool.
In conclusion, oxygen toxicity at 1.5 and 1.75 ATA in chronic neurologically injured patients is a certainty. Acute toxicity seems to occur more frequently at 1.75 ATA than 1.5 ATA. Chronic oxygen toxicity occurs earlier in the treatment course at 1.75 ATA than 1.5 ATA and more frequently after prolonged uninterrupted courses of HBOT or escalation of pressure from 1.5 to 1.75 or 2.0 ATA. Finally, a third syndrome is described of an acute withdrawal effect from HBOT after prolonged courses at 1.75 ATA that responds to additional HBOT at lower pressures. All three of these syndromes may have long-term detrimental effects.
References
- Harch PG, Neubauer RA (1999) Hyperbaric oxygen therapy in global cerebralischemia/anoxia and coma. In Jain KK (ed) Textbook of Hyperbaric Medicine, 3rdRevised Edition, Chapter 18. Hogrife & Huber Publishers, Seattle WA 1999: 31~-345.
- Hyperbaric Oxygen Therapy: 1999 Committee Report. Editor, N.B. Hampson. Undersea and Hyperbaric Medical Society, Kensington, MD.
- Marx RE, Johnson RP (1988), Problem Wounds in Oral and Maxillofacial Surgery: The Role of Hyperbaric Oxygen. In Davis JC and Hunt TK (eds.) Problem Wounds,The Role of Oxygen, Chapter 4. Elsevier Science Publishing Co., New York, NY 1988: 65-123.
- Siddiqui A, et al. Ischemic tissue oxygen capacitance after hyperbaric oxygen therapy: a new physiologic concept. Plast Reconstr Surg, 1995;99:148-155.
- Buras JA, et al. Hyperbaric oxygen down regulates ICAM-1 expression induced byhypoxia and hypoglycemia: the role of NOS. Am J Physiol Cell Physiol.2000;278:292-302.
- Reenstra WR, et al. Hyperbaric oxygen increases human dermal fibroblast expression of EGF-receptors (EGFR). Undersea & Hyperb Med, 1998b;25:54.
- Neubauer RA, et al. Enhancing”idling’ neurons. Lancet, 1990;335:542.
- Astrup J, Simon L (1981) Thresholds in cerebral ischemia — The ischemic penumbra. Stroke 12:6, 723-725.
- Hatch PG, et al. Low pressure hyperbaric oxygen therapy (LpHBOT) induces cerebrovascular changes and improves cognitive function in a rat traumatic brain injury (TBI) model. Undersea and Hyperbaric Med, 2001;28(Suppi):28.
- LewisRJ, WearsRL. AnlntroductiontotheBayesianAnalysisofClinicalTrials. AnnalsofEmergMed, August, 1993;22(8): 1328-1336.
- Clark JM (1993 ), Oxygen Toxicity. In Peter Bennett and David Elliott (eds.) The Physiology and Medicine of Diving, 4th Edition, Chapter 6. W. B. Sanriders Company, Ltd., London, England 1993: 121-169.
- Hatch PG, et al. The effect of HBOT tailing treatment on neurological residual and SPECT brain images in type II (cerebral) DCI/CAGE. Undersea and Hyperbaric Medicine, 1994;21(Suppi):22-23.
- Harch PG, et al. SPECT brain imaging in the diagnosis and treatment of type II decompression sickness. Undersea and Hyperbaric Medicine, 1992;19(Suppi):42.
- Hatch PG, et al. HMPAO SPECT Brain Imaging and Low Pressure HBOT in the Diagnosis and Treatment of Chronic Traumatic, Ischemic, Hypoxic, and Anoxic Encephalopathies. UnderseaHyperMed, 6/1994;21($uppl):30
- Barrett KF, Masel BE, Hatch PG, et al. Cerebral blood flow changes and cognitive improvement in chronic stable traumatic brain injuries treated with hyperbaric oxygen therapy. Neurol, April, 1998 ($uppl):A178-A179.
- Donald KW. Oxygen Poisoning in Man, Part II. British Med Journal, May 24, 1947; I: 712-717.
- Donald KW. Oxygen Poisoning in Man, Part I. British Med Journal, May 17, 1947; I: 667-672.
- Chavko M et al: Role of cerebral blood flow in seizures from hyperbaric oxygen exposure. Brain Research, 1998; 791:75-82.
- Bean JW et al ( 1972): Cerebral 02, CO2, regional cerebral vascular control, and hyperbaric oxygenation. J Applied Physiology May 1972; 32(5):650-657.
- Bergu GW, Tyssebotn I. Cerebral blood flow distribution and systemic haemodynamic changes after repeated hyperbaric oxygen exposures in rats. Europ J ApplPhysio, 1994; 69: 1-9.
21. Balentine JE (1982). Pathology of oxygen toxicity. Academic Press. New York.