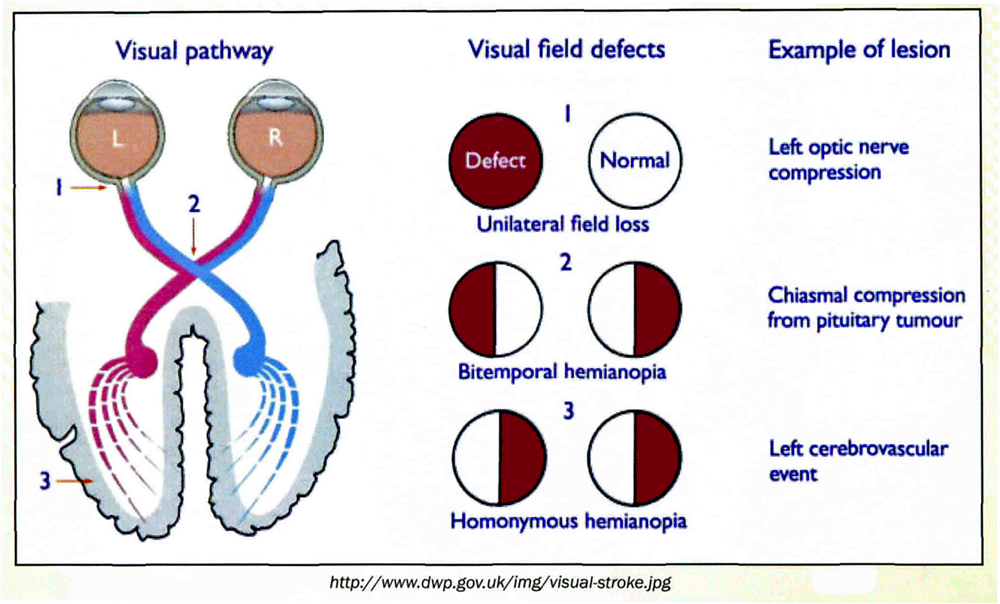
Central Retinal Artery Occlusion (CRAO) is a painless, severe, usually sudden onset loss of vision in the eye that is due to occlusion of the central retinal artery. Visual loss is in the range of light perception to counting fingers in 90% of cases. In primates, if vision isn’t restored within approximately 90-100 minutes, permanent injury to retinal cells occurs. In humans, however, the situation is more complicated and depends on retinal vascular anatomy. In patients with a cilioretinal artery that supplies part of the fovea, 10% of the population, 80% will have return of 20/50 vision. In the remainder of the population with CRAO, vision remains at the counting fingers to hand-motion level.
In the 1980s, the physician team at the JoEIIen Smith Hyperbaric Medicine Department in New Orleans, under the direction of Dr. Keith Van Meter, began to treat central retinal artery occlusion. The cases were referred by forward thinking retinal specialists who had read case reports of a small series of patients who had been treated with hyperbaric oxygen therapy. Eventually, we were referred every CRAO patient from a particular ophthalmology practice. Given the typically grim prognosis of these patients and the lack of substantive evidence that established a firm timeline beyond which HBOT would be ineffective, we treated every patient. We were able to achieve improvement in vision in the great majority of patients, despite delays to treatment beyond 12 hours. One of the key features of this treatment was an adaptive approach to dosing where we would decrease the HBOT dose from the traditional 2.4-2.8 ATA to as low as 1.5 ATA, as the patient plateaued at the higher pressures. In 2004, one of our hyperbaric fellows, Dr. Heather Murphy-Lavoie, reviewed our patient series and compared them to an age-matched control group from Charity Hospital, New Orleans. She presented the abstract at the 2004 UHMS meeting in Sydney, Australia. Based on this presentation, she was invited to submit an application to the UHMS HBOT Committee to add CRAO to the accepted indications list. That application was submitted and approved in 2008 with an American Heart Association Class lib level of evidence: “There is fair to good evidence to support its use with retrospective case series but no prospective randomized controlled trials. It is acceptable, safe, considered efficacious but lacks confirmation of efficacy by level 1 studies. There is no evidence of harm and consistently positive results. In addition, there are no alternative therapies with similar outcomes.”¹
The importance of the CRAO HBOT Committee approval lies in the implications for the future of additional occlusive vascular indications. CRAO is a rare condition. The impact of HBOT on individual cases is substantial and justifies the application of HBOT for this condition. However, the impact on society is minimal. What is more important is the scientific argument for HBOT that is based on the pathophysiology of CRAO, which is a sudden complete occlusion of arterial blood supply to nervous tissue. Sudden arterial occlusion is nearly identical pathophysiologically to the accepted indication of traumatic interruption of peripheral arterial supply and the Medicare indication of acute peripheral arterial insufficiency. CRAO is the same pathophysiology in the retinal circulation. In all three of these conditions, there is a period of complete or near complete interruption of blood supply which is followed by a period of reperfusion injury as circulation is re-established either surgically or by recanalization. The peripheral arterial interruptions/insufficiencies are better described in lay terms as “acute traumatic stroke” or “acute stroke of the arm or leg” while CRAO then is an “acute stroke of the eye.”
The analogy to “stroke” is important because HBOT is the use of greater than atmospheric pressure oxygen as a drug to treat basic disease processes. In all three of these conditions we are treating an underlying complete occlusion or interruption of blood supply to human tissue. Two of the tissues are outside the central nervous system and the third is in the central nervous system. The tissue and its location are somewhat irrelevant, but if we can now accept stroke of the eye based on Class lib evidence, it should expand our thinking to the broad range of pathophysiologically similar conditions characterized by gross cessation/interruption and then re-establishment of blood supply. Examples include hepatic artery occlusion (there are a number of references for HBOT treatment of this condition in the pediatric liver transplant literature), mesenteric artery occlusion, coronary artery occlusion, (acute myocardial infarction), the group of global ischemias (cardiac arrest, near-hanging, near-drowning), and of course, cerebral artery occlusion (stroke). Interestingly, HBOT in cerebral stroke is supported by over 35 animal and an equal number of human studies, some prospective and controlled, with the great majority at least meeting the Class lib or higher level of evidence. The following cases should stimulate your thinking of application of HBOT based on pathophysiology rather than diagnosis.
Reference List
1. Heather Murphy-Lavoie, Frank Butler and Catherine Hagan. Central Retinal Artery Occlusion. In: Gesell LB, Chair and Editor, Hyperbaric Oxygen Therapy Indications, 12th Edition. The Hyperbaric Oxygen Therapy Committee Report, Durham, NC: Undersea and Hyperbaric Medical Society, 2008. p.57-66.
Hyperbaric Oxygen Therapy for Central Retinal Occlusion
By Frank K. Butler, M.D., FAAO
Untreated central retinal artery occlusion (CRAO) usually results in permanent loss of vision in the affected eye unless a cilioretinal artery is present. (Hayreh 2005) Blindness is such a disabling condition that every reasonable therapeutic measure should be undertaken to prevent the loss of vision from becoming permanent. Most of the currently used treatments for CRAO are ineffective, but hyperbaric oxygen therapy {HBOT) may be useful in reversing vision loss in at least some cases of CRAO {Butler 2008, Murphy-Lavoie 2009, Butler 2007, Henderson 2004, Weinberger 2002, Beiran 2001, Phillips 1999, Li 1996, Yotsukura 1993, Beiran 1993, Hertzog 1992, Pallotta 1978, Stone 1977).
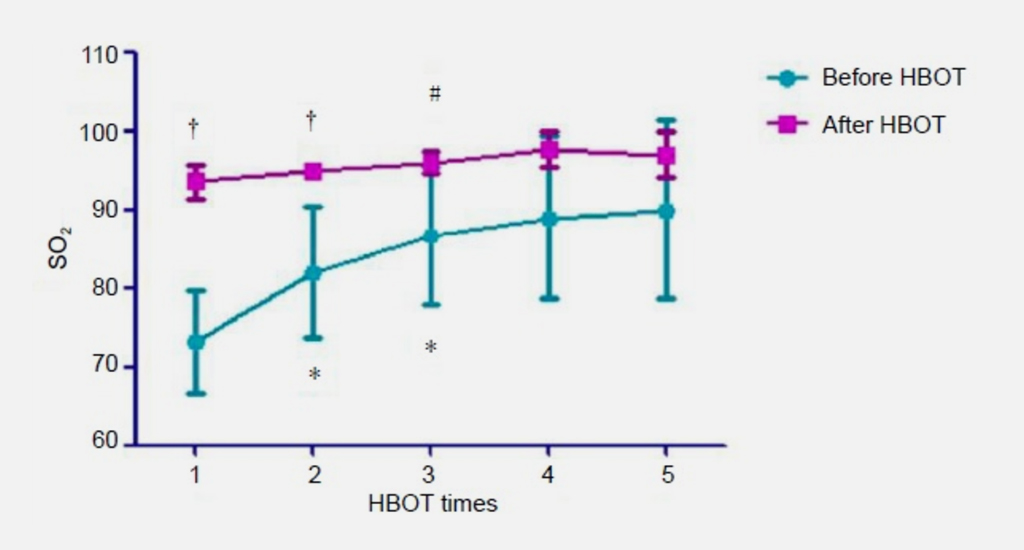
The outer portion of the retina {over half of the total thickness) is oxygenated by the choroid vessels even when breathing air at 1 ATA {Li 1996, Landers 1978, Patz 1955). HBOT is effective in CRAO if the treatment is begun early enough because the increased partial pressure of oxygen allows the entire retina to be oxygenated by diffusion of oxygen from the choroidal vessels despite the retinal vasoconstriction that occurs with hyperoxia (Landers 1978, Frayser 1967, James 1985, Jampol 1987, Dollery 1969, Saltzman 1965, Patz 1955). Clinicians may note that HBOT results in retinal artery vasoconstriction and reduced blood flow in those vessels. While this is true, choroidal blood flow is not significantly affected by hyperbaric oxygen, (Li 1996, Yu 2005) allowing blood flow in that circulation to continue at pre-HBOT levels. CRAO has recently been added to the approved list of indications for HBOT by the HBO committee of the Undersea and Hyperbaric Medical Society {Murphy-Lavoie 2009).
Preventing loss of vision in an eye that has suffered a CRAO through the use of hyperoxic therapy requires three things:
- The HBOT must be undertaken before irreversible damage to the retina has been sustained -HBOT begun after 24 hours is unlikely to be successful in CRAO.
- The arterial occlusion must be at the level of the central retinal artery and not at the ophthalmic artery level, which precludes flow to the choroidal vessels as well.
- Hyperbaric or supplemental oxygen sufficient to 1 maintain retinal viability is maintained, at least intermittently, until the CRA recanalizes (typically hours to days -Duker 1988) and the inner layers of the retina are again adequately oxygenated through the retinal vessels. Optimized post-initial HBOT management for CRAO patients who have responded successfully to initial HBOT remains to be defined.
Reference List
Beiran I, Goldenberg I, Adir Y, Tamir A, Shupak A, Miller B. Early hyperbaric oxygen therapy for retinal artery occlusion. Eur J Ophthalmol2001;11:345-50.
Beiran I, Reissman P, Scharf J, Nahum Z, Miller B. Hyperbaric oxygenation combined with nifedipine treatment for recent-onset retinal artery occlusion. Eur J Ophthalmol1993;3:89-94.
Butler FK, Hagan C, Murphy-Lavoie H: Hyperbaric Oxygen Therapy and the Eye. Undersea Hyperb Med 2008;35:333-387
Butler FK. The Eye in the Wilderness. In: Wilderness Medicine; Auerbach PS, ed: StLouis, Mosby; Fifth Edition, 2007. Do//ery CT, Bulpitt CJ, Kohner EM. Oxygen supply to the retina from the retinal and choroidal circulatons at normal and increased arterial oxygen tensions. Invest Ophthalmol 1969;8:588-594.
Duker JS, Brown GC. Recovery following acute obstruction of the retinal and choroidal circulations. Retina 1988;8:257-260.
Frayser R, Saltzman HA, Anderson 8, Hickam JB, Sieker. The effect of hyperbaric oxygenation on retinal circulation. Arch Ophthalmol 1967; 77:265-269.
Hayreh SS, Zimmerman MB. Central retinal artery occlusion: Visual outcome. Am J Ophthalmol 2005;140:376-391.
Henderson LT, Slade JB. Central retinal artery occlusion and hyperbaric oxygen therapy. Undersea Hyperb Med 2004:31:309.
(abstract) Hertzog LM, Meyer GW, Carson S, Strauss MB, Hart GB. Central retinal artery occlusion treated with hyperbaric oxygen. J Hyperbaric Medicine 1992;7:33-42.
James PB. Hyperbaric oxygen and retinal vascular changes: Hyperbaric oxygen and retinal vascular changes. Med J Aust 1985;142:163-164.
Landers MB. Retinal oxygenation via the choroidal circulation. Trans Am Ophthalmol Soc 1978;76:528-556.
Li HK, Dejean BJ, Tang RA. Reversal of visual loss with hyperbaric oxygen treatment in a patient with Susac Syndrome. Ophthalmology 1996;103: 2091-2098.